Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.40397 Ge Liu 1 , Yantao Bao 1 , Chaohua Liu 2 , Qinchang Zhu 3 , Lin Zhao 2 , Xiaopeng Lu 1 , Qian Zhu 1 , Yafei Lv 1 , Feng Bai 4 , He Wen 1 , Yujie Sun 5 , Wei-Guo Zhu 1, 6
|
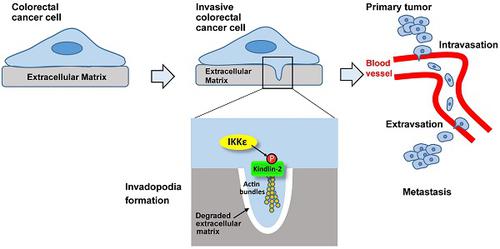
Invadopodia formation is a key driver of cancer metastasis. The noncanonical IkB-related kinase IKKε has been implicated in cancer metastasis, but its roles in invadopodia formation and colorectal cancer (CRC) metastasis are unclear.
Methods: Immunofluorescence, gelatin-degradation assay, wound healing assay and transwell invasion assay were used to determine the influence of IKKε over-expression, knockdown and pharmacological inhibition on invadopodia formation and the migratory and invasive capacity of CRC cells in vitro. Effects of IKKε knockdown or pharmacological inhibition on CRC metastasis were examined in mice. Immunohistochemistry staining was used to detect expression levels of IKKε in CRC patient tissues, and its association with prognosis in CRC patients was also analyzed. Immunoprecipitation, western blotting and in vitro kinase assay were constructed to investigate the molecular mechanisms.
Results: IKKε co-localizes with F-actin and the invadopodia marker Tks5 at the gelatin-degrading sites of CRC cells. Genetic over-expression/knockdown or pharmacological inhibition of IKKε altered invadopodia formation and the migratory and invasive capacity of CRC cells in vitro. In vivo, knockdown or pharmacological inhibition of IKKε significantly suppressed metastasis of CRC cells in mice. IKKε knockdown also inhibited invadopodia formation in vivo. Clinical investigation of tumor specimens from 191 patients with CRC revealed that high IKKε expression correlates with metastasis and poor prognosis of CRC. Mechanistically, IKKε directly binds to and phosphorylates kindlin-2 at serine 159; this effect mediates the IKKε-induced invadopodia formation and promotion of CRC metastasis.
Conclusions: We identify IKKε as a novel regulator of invadopodia formation and a unique mechanism by which IKKε promotes the metastasis of CRC. Our study suggests that IKKε is a potential target to suppress CRC metastasis.
中文翻译:

IKKε使kindlin-2磷酸化,从而诱导小脚印形成并促进结直肠癌转移。
虫足的形成是癌症转移的关键驱动力。非典型的IkB相关激酶IKKε已参与癌症转移,但尚不清楚其在侵袭性伪足形成和结直肠癌(CRC)转移中的作用。
方法:采用免疫荧光法,明胶降解法,伤口愈合法和transwell侵袭法测定IKKε过表达,敲除和药理抑制作用对体外培养的侵袭性伪足形成及迁移能力的影响。在小鼠中检查了IKKε敲除或药理学抑制作用对CRC转移的影响。免疫组织化学染色用于检测结直肠癌患者组织中IKKε的表达水平,并分析其与结直肠癌患者预后的关系。建立了免疫沉淀,蛋白质印迹和体外激酶测定的方法,以研究其分子机制。
结果:IKKε与F-肌动蛋白和invadopodia标记Tks5共定位在CRC细胞的明胶降解位点。IKKε的基因过表达/抑制或药理抑制作用改变了体外侵袭足细胞的形成和迁移和侵袭能力。在体内,IKKε的敲低或药理学抑制作用显着抑制了小鼠CRC细胞的转移。IKKε击倒还抑制体内侵袭伪足的形成。对191例CRC患者的肿瘤标本进行的临床研究表明,高IKKε表达与CRC的转移和预后不良有关。从机理上讲,IKKε直接与丝氨酸159上的kindlin-2结合并使其磷酸化;这种作用介导了IKKε诱导的伪足形成和促进CRC转移。
结论:我们确定IKKε是新的侵染性伪足形成调节剂,并且是IKKε促进CRC转移的独特机制。我们的研究表明,IKKε是抑制CRC转移的潜在靶标。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号