当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphdiyne-Supported Single Iron Atom: A Promising Electrocatalyst for Carbon Dioxide Electroreduction into Methane and Ethanol
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.jpcc.9b11649 Xin Liu 1 , Zhongxu Wang 1 , Yu Tian 2 , Jingxiang Zhao 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.jpcc.9b11649 Xin Liu 1 , Zhongxu Wang 1 , Yu Tian 2 , Jingxiang Zhao 1
Affiliation
|
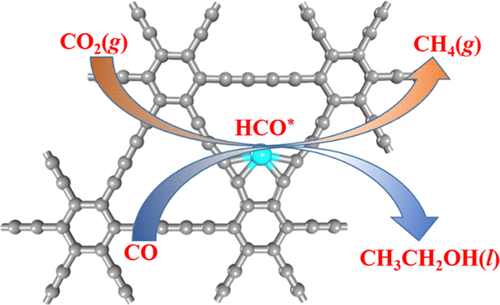
Electrochemical reduction of carbon dioxide (CO2ER) to high-energy-density multicarbon products is a quite promising technique for large-scale renewable energy storage, for which searching for stable, inexpensive, and efficient catalysts is a key scientific issue. In this work, the potential of an experimentally available single iron (Fe) atom supported on graphdiyne (Fe/GDY) as the CO2ER catalyst was explored by means of density functional theory (DFT) computations. Our results revealed that Fe/GDY exhibits high stability due to the strong hybridization between the Fe 3d orbitals and the C 2p orbitals of GDY. Interestingly, due to the small limiting potential of −0.43 V, the anchored Fe atom can effectively reduce CO2 to CH4 along the following pathway: CO2 → HCOO* → HCOOH* → HCO* → H2CO* → H3CO* → O* + CH4 → OH* → H2O, in which the hydrogenation of HCOOH* to HCO* is the potential-determining step. Furthermore, the unsaturated HCO* species on Fe/GDY can provide an active site for further coupling with CO to generate C2H5OH with a small activation energy for C–C coupling. Our theoretical results not only propose a new approach to CO2ER to C2 products on a single-site catalyst but also further widen the potential applications of GDY.
中文翻译:

石墨二炔支持的单铁原子:一种有望将二氧化碳电还原为甲烷和乙醇的电催化剂
将二氧化碳(CO 2 ER)电化学还原为高能量密度的多碳产品是大规模可再生能源存储中非常有前途的技术,为此寻求稳定,廉价和高效的催化剂是关键的科学问题。在这项工作中,通过密度泛函理论(DFT)计算,探索了实验上可用的负载在石墨二炔(Fe / GDY)上的单个铁(Fe)原子作为CO 2 ER催化剂的潜力。我们的结果表明,Fe / GDY表现出很高的稳定性,这是由于GDY的Fe 3d轨道和C 2p轨道之间的强杂交。有趣的是,由于固定电位为-0.43 V,因此固定的Fe原子可以有效地将CO 2还原为CH 4遵循以下路径:CO 2 →HCOO *→HCOOH *→HCO *→H 2 CO *→H 3 CO *→O * + CH 4 →OH *→H 2 O,其中HCOOH *加氢为HCO *是确定电位的步骤。此外,Fe / GDY上的不饱和HCO *物种可以为进一步与CO偶联提供一个活性位点,从而生成具有较小活化能的C 2 H 5 OH进行C-C偶联。我们的理论结果不仅提出了在单中心催化剂上将CO 2 ER转化为C 2产物的新方法,而且进一步拓宽了GDY的潜在应用范围。
更新日期:2020-02-04
中文翻译:

石墨二炔支持的单铁原子:一种有望将二氧化碳电还原为甲烷和乙醇的电催化剂
将二氧化碳(CO 2 ER)电化学还原为高能量密度的多碳产品是大规模可再生能源存储中非常有前途的技术,为此寻求稳定,廉价和高效的催化剂是关键的科学问题。在这项工作中,通过密度泛函理论(DFT)计算,探索了实验上可用的负载在石墨二炔(Fe / GDY)上的单个铁(Fe)原子作为CO 2 ER催化剂的潜力。我们的结果表明,Fe / GDY表现出很高的稳定性,这是由于GDY的Fe 3d轨道和C 2p轨道之间的强杂交。有趣的是,由于固定电位为-0.43 V,因此固定的Fe原子可以有效地将CO 2还原为CH 4遵循以下路径:CO 2 →HCOO *→HCOOH *→HCO *→H 2 CO *→H 3 CO *→O * + CH 4 →OH *→H 2 O,其中HCOOH *加氢为HCO *是确定电位的步骤。此外,Fe / GDY上的不饱和HCO *物种可以为进一步与CO偶联提供一个活性位点,从而生成具有较小活化能的C 2 H 5 OH进行C-C偶联。我们的理论结果不仅提出了在单中心催化剂上将CO 2 ER转化为C 2产物的新方法,而且进一步拓宽了GDY的潜在应用范围。

































 京公网安备 11010802027423号
京公网安备 11010802027423号