当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel pyrazole sulfonamide derivatives as dual COX-2/5-LOX inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.ejmech.2020.112066 Ehab M Gedawy 1 , Asmaa E Kassab 2 , Ahmed M El Kerdawy 3
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.ejmech.2020.112066 Ehab M Gedawy 1 , Asmaa E Kassab 2 , Ahmed M El Kerdawy 3
Affiliation
|
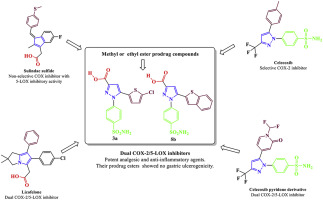
The current therapeutic demand focuses more on the discovery of safer NSAIDs rather than exploring more potent alternatives. The dual COX-2/5-LOX inhibition is a promising strategy for designing compounds with an enhanced efficacy, reduced side-effects and a broader anti-inflammatory spectrum in comparison to classical NSAIDs. In the present study, a hybridization strategy was adopted to combine the binding features of the non-selective COX inhibitor "sulindac" and the selective COX-2 inhibitor "celecoxib" which show 5-LOX inhibitory activity with that of licofelone and a celecoxib pyridone analogue which show dual COX-2/5-LOX inhibitory activity to design new series of pyrazole sulfonamide derivatives which, by design, should possess dual COX-2/5-LOX inhibitory activity. All the newly synthesized compounds were initially tested for their potential analgesic activity, then candidates that showed potential analgesic activity, were selected for the subsequent anti-inflammatory activity evaluation, as well as, ulcerogenicity testing. Moreover, in vitro assessment of their COX-1, COX-2 and 5-LOX inhibitory activities were performed. The benzothiophen-2-yl pyrazole carboxylic acid derivative 5b showed the most potent analgesic and anti-inflammatory activities surpassing that of celecoxib and indomethacin. It showed potent COX-1, COX-2 and 5-LOX inhibitory activity with IC50 of 5.40, 0.01 and 1.78 μM, respectively, showing a selectivity index of 344.56 that was much better than the used reference standards and its parent compounds, confirming its selectivity towards COX-2 over COX-1. The prodrug ester derivatives 6c and 6d showed equipotent activity to their parent compound 5b with no gastric ulcerogenicity. Molecular docking simulations confirmed that the newly synthesized compounds possess the structural features required for binding to the target enzymes COX-2 and 5-LOX.
中文翻译:

新型吡唑磺酰胺衍生物作为双COX-2 / 5-LOX抑制剂的设计,合成和生物学评估。
当前的治疗需求更多地集中在发现更安全的非甾体抗炎药,而不是探索更有效的替代品。与经典的NSAID相比,双重COX-2 / 5-LOX抑制是设计具有增强的功效,减少的副作用和更广的抗炎谱的化合物的有前途的策略。在本研究中,采用杂交策略来结合非选择性COX抑制剂“舒林酸”和选择性COX-2抑制剂“塞来昔布”的结合特征,后者具有5-LOX抑制活性,并具有licofelone和celecoxib吡啶酮的抑制活性。具有双COX-2 / 5-LOX抑制活性的类似物,以设计新系列的吡唑磺酰胺衍生物,该吡唑磺酰胺衍生物经设计应具有双COX-2 / 5-LOX抑制活性。首先测试所有新合成的化合物的潜在镇痛活性,然后选择显示出潜在镇痛活性的候选药物用于随后的抗炎活性评估以及致溃疡性测试。此外,对其COX-1,COX-2和5-LOX抑制活性进行了体外评估。苯并噻吩-2-基吡唑羧酸衍生物5b的最强镇痛和抗炎活性超过了塞来昔布和消炎痛。它显示出强效的COX-1,COX-2和5-LOX抑制活性,IC50分别为5.40、0.01和1.78μM,显示出344.56的选择性指数,远优于所用的参考标准品及其母体化合物,证实了其对COX-2的选择性高于对COX-1的选择性。前药酯衍生物6c和6d显示出与其母体化合物5b同等的活性,没有胃溃疡性。分子对接模拟证实,新合成的化合物具有与目标酶COX-2和5-LOX结合所需的结构特征。
更新日期:2020-01-17
中文翻译:

新型吡唑磺酰胺衍生物作为双COX-2 / 5-LOX抑制剂的设计,合成和生物学评估。
当前的治疗需求更多地集中在发现更安全的非甾体抗炎药,而不是探索更有效的替代品。与经典的NSAID相比,双重COX-2 / 5-LOX抑制是设计具有增强的功效,减少的副作用和更广的抗炎谱的化合物的有前途的策略。在本研究中,采用杂交策略来结合非选择性COX抑制剂“舒林酸”和选择性COX-2抑制剂“塞来昔布”的结合特征,后者具有5-LOX抑制活性,并具有licofelone和celecoxib吡啶酮的抑制活性。具有双COX-2 / 5-LOX抑制活性的类似物,以设计新系列的吡唑磺酰胺衍生物,该吡唑磺酰胺衍生物经设计应具有双COX-2 / 5-LOX抑制活性。首先测试所有新合成的化合物的潜在镇痛活性,然后选择显示出潜在镇痛活性的候选药物用于随后的抗炎活性评估以及致溃疡性测试。此外,对其COX-1,COX-2和5-LOX抑制活性进行了体外评估。苯并噻吩-2-基吡唑羧酸衍生物5b的最强镇痛和抗炎活性超过了塞来昔布和消炎痛。它显示出强效的COX-1,COX-2和5-LOX抑制活性,IC50分别为5.40、0.01和1.78μM,显示出344.56的选择性指数,远优于所用的参考标准品及其母体化合物,证实了其对COX-2的选择性高于对COX-1的选择性。前药酯衍生物6c和6d显示出与其母体化合物5b同等的活性,没有胃溃疡性。分子对接模拟证实,新合成的化合物具有与目标酶COX-2和5-LOX结合所需的结构特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号