当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Syntheses of Spiro[indoline-3,4'-pyridin]-2-yl Carbamates via AgOTf/Ph3P-Catalyzed Tandem Cyclizations of Tryptamine-Ynesulfonamides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.joc.9b02839 Guoduan Liang 1, 2, 3 , Yadong Pang 1, 2, 3 , Yanjun Ji 1, 3 , Kaitong Zhuang 1, 3 , Linji Li 1, 3 , Fukai Xie 1, 2, 3 , Lu Yang 1, 2, 3 , Maosheng Cheng 1, 3 , Bin Lin 1, 3 , Yongxiang Liu 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.joc.9b02839 Guoduan Liang 1, 2, 3 , Yadong Pang 1, 2, 3 , Yanjun Ji 1, 3 , Kaitong Zhuang 1, 3 , Linji Li 1, 3 , Fukai Xie 1, 2, 3 , Lu Yang 1, 2, 3 , Maosheng Cheng 1, 3 , Bin Lin 1, 3 , Yongxiang Liu 1, 2, 3
Affiliation
|
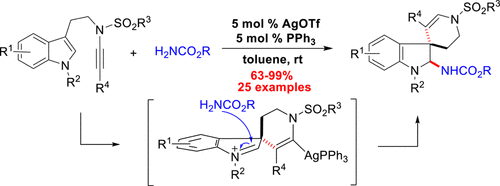
Spiro[indoline-3,4'-piperidine] is a significant structural scaffold in numerous polycyclic indole alkaloids with a variety of bioactivities. In this study, a synthetic strategy was developed to access spiro[indoline-3,4'-pyridin]-2-yl carbamate via an AgOTf/PPh3-catalyzed tandem cyclization of tryptamine-ynesulfonamides. The unique feature of this strategy is the efficient intermolecular capturing of the in situ generated spiroindoleninium intermediates with carbamates, leading to the diastereoselective syntheses of spiro[indoline-3,4'-pyridin]-2-yl carbamate derivatives. A broad scope of this cyclization was demonstrated by a variety of tryptamine-ynesulfonamide substrates and several carbamates. A plausible mechanism of this reaction was proposed.
中文翻译:

通过AgOTf / Ph3P催化的色胺-Ynesulfonamides的串联环化非对映选择性合成氨基[吲哚啉-3,4'-吡啶] -2-基氨基甲酸酯。
螺[吲哚啉-3,4'-哌啶]是许多具有多种生物活性的多环吲哚生物碱中的重要结构骨架。在这项研究中,开发了一种合成策略,可通过AgOTf / PPh3催化的类胰蛋白酶-炔磺酰胺的串联环化来获得螺[吲哚啉-3,4'-吡啶] -2-氨基甲酸酯。该策略的独特之处在于可以有效地分子间捕获具有氨基甲酸酯的原位生成的螺环己二烯中间体,从而导致螺环[吲哚啉-3,4'-吡啶]]-2-基氨基甲酸酯衍生物的非对映选择性合成。多种色胺-氨磺酰胺底物和几种氨基甲酸酯证明了这种环化作用的广泛范围。提出了该反应的合理机制。
更新日期:2020-01-29
中文翻译:

通过AgOTf / Ph3P催化的色胺-Ynesulfonamides的串联环化非对映选择性合成氨基[吲哚啉-3,4'-吡啶] -2-基氨基甲酸酯。
螺[吲哚啉-3,4'-哌啶]是许多具有多种生物活性的多环吲哚生物碱中的重要结构骨架。在这项研究中,开发了一种合成策略,可通过AgOTf / PPh3催化的类胰蛋白酶-炔磺酰胺的串联环化来获得螺[吲哚啉-3,4'-吡啶] -2-氨基甲酸酯。该策略的独特之处在于可以有效地分子间捕获具有氨基甲酸酯的原位生成的螺环己二烯中间体,从而导致螺环[吲哚啉-3,4'-吡啶]]-2-基氨基甲酸酯衍生物的非对映选择性合成。多种色胺-氨磺酰胺底物和几种氨基甲酸酯证明了这种环化作用的广泛范围。提出了该反应的合理机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号