当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Polymerized Dopamine-Decorated Au NPs and Coordinated with Fe-MOF as a Dual Binding Sites and Dual Signal-Amplifying Electrochemical Aptasensor for the Detection of CEA.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acsami.9b19161 Jifan Li 1, 2 , Lei Liu 2 , Yongjian Ai 1 , Yang Liu 1 , Hongbin Sun 2 , Qionglin Liang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acsami.9b19161 Jifan Li 1, 2 , Lei Liu 2 , Yongjian Ai 1 , Yang Liu 1 , Hongbin Sun 2 , Qionglin Liang 1
Affiliation
|
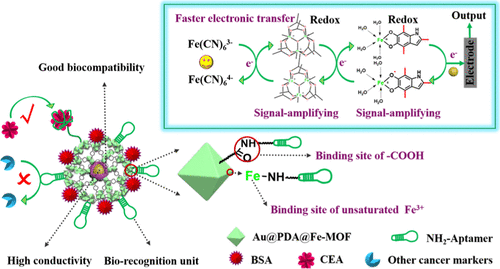
Fabrication of functional electrochemical biosensor is a hot topic; however, precise and sensitive cancer detection in early clinical diagnosis is still a great challenge. Continuous efforts have been devoted to explore functional materials for this issue. In this work, we developed a dual binding sites and dual signal-amplifying electrochemical aptasensor of self-polymerized dopamine-decorated Au and coordinated with Fe-MOF (Au@PDA@Fe-MOF) for the detection of carcinoembryonic antigen (CEA). Remarkably, Au@PDA@Fe-MOF features high sensitivity, multiple active sites, good biocompatibility, and excellent selectivity, which is attributed to abundant -COOH in porous Fe-MOF and unsaturated Fe3+ sites on the surface of Fe-MOF as the active binding sites grafting more NH2-functionalized CEA-specific aptamer and redox PDA and Fe-MOF accelerating the movement of electrons for dual signal amplifying. Meanwhile, the electrochemical aptasensor shows favorable repeatability with 1.82% relative standard deviation (RSD) under five independent aptasensors and strong stability with only 3.3% degradation after 12 days of storage. In addition, the aptasensor has wide CEA detection range from 1 fg mL-1 to 1 μg mL-1 with a low detection limit of 0.33 fg mL-1 (S/N = 3). Furthermore, the aptasensor is feasible for accurate and quantitative detection of CEA in serum samples with RSD below 2.32%. The satisfying results demonstrate promising applications of the CEA aptasensor in practical sample analysis and lay an important foundation for other biomarker detection in early clinical diagnosis.
中文翻译:

自聚合多巴胺修饰的金纳米颗粒,并与铁-MOF配合作为双结合位点和双信号放大电化学传感器,用于检测CEA。
功能性电化学生物传感器的制备是一个热门话题。然而,在早期临床诊断中精确而敏感的癌症检测仍然是一个巨大的挑战。为了解决该问题,已经进行了持续的努力来探索功能材料。在这项工作中,我们开发了自聚合的多巴胺修饰的Au的双结合位点和双信号放大电化学适体传感器,并与Fe-MOF(Au @ PDA @ Fe-MOF)配合用于检测癌胚抗原(CEA)。值得注意的是,Au @ PDA @ Fe-MOF具有高灵敏度,多个活性位点,良好的生物相容性和出色的选择性,这归因于多孔Fe-MOF中大量的-COOH和Fe-MOF表面上的不饱和Fe3 +位点,因为它们的活性结合位点接枝了更多的NH2官能化CEA特异性适体和氧化还原PDA,并且Fe-MOF加速了双信号放大。同时,电化学适体在五个独立的适体下显示出良好的可重复性,相对标准偏差(RSD)为1.82%,并且在储存12天后具有仅3.3%降解的强稳定性。此外,适体传感器的CEA检测范围很广,从1 fg mL-1到1μgmL-1,检测限很低,为0.33 fg mL-1(S / N = 3)。此外,适体传感器对于RSD低于2.32%的血清样品中CEA的准确和定量检测是可行的。
更新日期:2020-01-26
中文翻译:

自聚合多巴胺修饰的金纳米颗粒,并与铁-MOF配合作为双结合位点和双信号放大电化学传感器,用于检测CEA。
功能性电化学生物传感器的制备是一个热门话题。然而,在早期临床诊断中精确而敏感的癌症检测仍然是一个巨大的挑战。为了解决该问题,已经进行了持续的努力来探索功能材料。在这项工作中,我们开发了自聚合的多巴胺修饰的Au的双结合位点和双信号放大电化学适体传感器,并与Fe-MOF(Au @ PDA @ Fe-MOF)配合用于检测癌胚抗原(CEA)。值得注意的是,Au @ PDA @ Fe-MOF具有高灵敏度,多个活性位点,良好的生物相容性和出色的选择性,这归因于多孔Fe-MOF中大量的-COOH和Fe-MOF表面上的不饱和Fe3 +位点,因为它们的活性结合位点接枝了更多的NH2官能化CEA特异性适体和氧化还原PDA,并且Fe-MOF加速了双信号放大。同时,电化学适体在五个独立的适体下显示出良好的可重复性,相对标准偏差(RSD)为1.82%,并且在储存12天后具有仅3.3%降解的强稳定性。此外,适体传感器的CEA检测范围很广,从1 fg mL-1到1μgmL-1,检测限很低,为0.33 fg mL-1(S / N = 3)。此外,适体传感器对于RSD低于2.32%的血清样品中CEA的准确和定量检测是可行的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号