当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Systematic Investigation into the Formation of Significant Amounts of Unknown Impurity during Scale-up of NaBH4–I2 Mediated Reduction of Nitro-Amide Intermediate of Mirabegron#
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.oprd.9b00365 Dattatray G. Deshmukh 1 , Mukund N. Bangal 1 , Kaustubh A. Kalawade 1 , Vijayavitthal T. Mathad 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.oprd.9b00365 Dattatray G. Deshmukh 1 , Mukund N. Bangal 1 , Kaustubh A. Kalawade 1 , Vijayavitthal T. Mathad 1
Affiliation
|
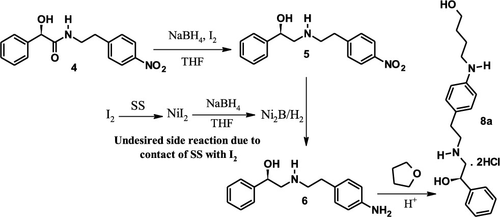
After successful development of a manufacturing process for the Mirabegron (1) at the laboratory scale, a muddled situation was aroused during the scale-up batches, wherein sodium borohydride-iodine (NaBH4–I2) mediated reduction of nitro-amide 4 ended up with substantial amounts (∼10%) of unspecified impurity in the nitro-amine intermediate 5. On the basis of the structure elucidation and meticulous investigation, a reaction path for its genesis during the process was identified and an efficient mechanism proposed to arrest its formation. In-situ generated nickel boride (Ni2B) due to reaction of NiI2 (corrosion product) with NaBH4 followed by electrophilic attack of THF (solvent) was found to be the reason for the formation of impurity (8a). Execution of subsequent batches with proper controls arrested this impurity and successfully provided the Mirabegron with the desired quality.
中文翻译:

期间加入NaBH的规模扩大的不明杂质的系统的调查显着的形成金额4 -I 2的Mirabegron硝基酰胺中间体的介导还原#
在实验室规模成功开发了Mirabegron(1)的制造工艺之后,在批量生产过程中出现了混乱的局面,其中硼氢化钠-碘(NaBH 4 -I 2)介导的硝基酰胺4还原结束在硝基胺中间体5中添加大量(〜10%)未指明的杂质。通过对结构的阐明和细致的研究,确定了其在生成过程中的反应路径,并提出了阻止其形成的有效机制。NiI 2(腐蚀产物)与NaBH 4的反应原位生成的硼化镍(Ni 2 B)随后发现THF(溶剂)的亲电腐蚀是形成杂质(8a)的原因。通过适当控制执行后续批次,可以制止这种杂质,并成功地为Mirabegron提供了所需的质量。
更新日期:2020-01-22
中文翻译:

期间加入NaBH的规模扩大的不明杂质的系统的调查显着的形成金额4 -I 2的Mirabegron硝基酰胺中间体的介导还原#
在实验室规模成功开发了Mirabegron(1)的制造工艺之后,在批量生产过程中出现了混乱的局面,其中硼氢化钠-碘(NaBH 4 -I 2)介导的硝基酰胺4还原结束在硝基胺中间体5中添加大量(〜10%)未指明的杂质。通过对结构的阐明和细致的研究,确定了其在生成过程中的反应路径,并提出了阻止其形成的有效机制。NiI 2(腐蚀产物)与NaBH 4的反应原位生成的硼化镍(Ni 2 B)随后发现THF(溶剂)的亲电腐蚀是形成杂质(8a)的原因。通过适当控制执行后续批次,可以制止这种杂质,并成功地为Mirabegron提供了所需的质量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号