当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of thieno[2,3-d]pyrimidin-4(3H)-one derivatives as a new class of ROCK inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.bmcl.2020.126966 Zhuang Miao 1 , Yu-Meng Sun 2 , Lan-Ying Zhao 1 , Yue-Shan Li 1 , Yi-Fei Wang 2 , Jin-Shan Nan 1 , Ze-En Qiao 1 , Lin-Li Li 2 , Sheng-Yong Yang 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.bmcl.2020.126966 Zhuang Miao 1 , Yu-Meng Sun 2 , Lan-Ying Zhao 1 , Yue-Shan Li 1 , Yi-Fei Wang 2 , Jin-Shan Nan 1 , Ze-En Qiao 1 , Lin-Li Li 2 , Sheng-Yong Yang 1
Affiliation
|
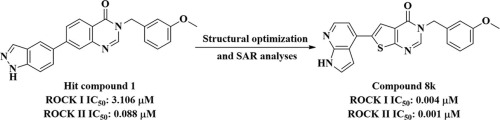
Herein, we report the discovery of a series of thieno[2,3-d]pyrimidin-4(3H)-one derivatives as a new class of ROCK inhibitors. Structure-activity relationship studies of these compounds led to the identification of the most potent compound, 3-(3-methoxybenzyl)-6-(1H-pyrrolo[2,3-b]pyridin-4-yl)thieno[2,3-d]pyrimidin-4(3H)-one (8k), which showed IC50 values of 0.004 μM and 0.001 μM against ROCK Ⅰ and ROCK Ⅱ, respectively. In vitro, 8k significantly reduced the phosphorylation level of ROCK downstream signaling protein and induce changes in cell morphology and migration. Overall, this study provides a promising lead compound for drug discovery targeting ROCKs.
中文翻译:

硫代[2,3-d]嘧啶-4(3H)-一衍生物的发现作为一类新型的ROCK抑制剂。
在本文中,我们报告了一系列噻吩[2,3-d]嘧啶-4(3H)-一衍生物作为一类新的ROCK抑制剂的发现。这些化合物的结构活性关系研究导致最有效的化合物,3-(3-甲氧基苄基)-6-(1H-吡咯并[2,3-b]吡啶-4-基)噻吩并[2,3]的鉴定-d] pyrimidin-4(3H)-one(8k),对ROCKⅠ和ROCKⅡ的IC50值分别为0.004μM和0.001μM。在体外,8k显着降低了ROCK下游信号蛋白的磷酸化水平,并诱导细胞形态和迁移的改变。总体而言,这项研究为靶向ROCKs的药物发现提供了有希望的先导化合物。
更新日期:2020-01-15
中文翻译:

硫代[2,3-d]嘧啶-4(3H)-一衍生物的发现作为一类新型的ROCK抑制剂。
在本文中,我们报告了一系列噻吩[2,3-d]嘧啶-4(3H)-一衍生物作为一类新的ROCK抑制剂的发现。这些化合物的结构活性关系研究导致最有效的化合物,3-(3-甲氧基苄基)-6-(1H-吡咯并[2,3-b]吡啶-4-基)噻吩并[2,3]的鉴定-d] pyrimidin-4(3H)-one(8k),对ROCKⅠ和ROCKⅡ的IC50值分别为0.004μM和0.001μM。在体外,8k显着降低了ROCK下游信号蛋白的磷酸化水平,并诱导细胞形态和迁移的改变。总体而言,这项研究为靶向ROCKs的药物发现提供了有希望的先导化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号