当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthetic Mechanism of Lanosterol: A Completed Story
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acscatal.9b05221 Hongjuan Diao 1 , Nanhao Chen 1, 2 , Kai Wang 1 , Fan Zhang 1 , Yong-Heng Wang 1, 3 , Ruibo Wu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acscatal.9b05221 Hongjuan Diao 1 , Nanhao Chen 1, 2 , Kai Wang 1 , Fan Zhang 1 , Yong-Heng Wang 1, 3 , Ruibo Wu 1
Affiliation
|
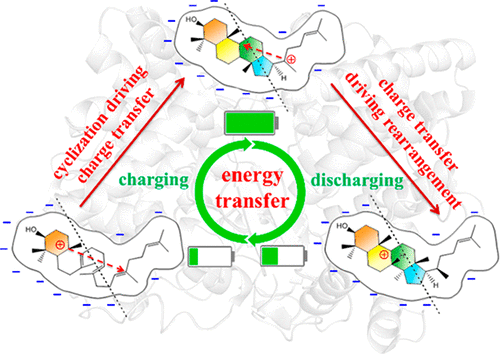
The remarkable biochemical conversion of acyclic 2,3-oxidosqualene to tetracyclic lanosterol (the common precursor to cholesterol and its relatives) with seven stereocenters constructed, catalyzed by oxidosqualene cyclase (OSC), has fascinated chemists for over a half century. Although many experimental and theoretical efforts have been reported, most of the studies focused on the initial cyclization while the subsequent cascade carbocation rearrangement and deprotonation were ignored, which determine the regio- and stereochemistry of the final product lanosterol associated with the function-related structure domains of cholesterol and its relatives. Herein we continue our previous works on the cyclization (Angew. Chem., Int. Ed.2015, 54, 8693–8696); the mechanistic details of the remaining part of lanosterol biosynthesis, involving three hydride shifts, two methyl shift and one deprotonation, were investigated by quantum mechanical/molecular mechanical (QM/MM) molecular dynamics (MD) simulations. We obtained the complete free energy profile for lanosterol biosynthesis, which is thermodynamically and kinetically reasonable for the sole formation of the desired product with byproducts effectively avoided. We identified some key factors such as electrostatic interactions and CH···π interactions that facilitate migration of the carbocation. We also identified the direct deprotonation precursor, intermediate I, which has been controversial in previous studies. More importantly, we found that the enzyme mediates the energy transfer from the initial cyclization to the subsequent rearrangements through the electric field of the active pocket, which guarantees the fidelity of the enzyme catalysis.
中文翻译:

羊毛甾醇的生物合成机制:完整的故事
无环2,3-氧化角鲨烯到四环羊毛甾醇(胆固醇及其亲戚的常见前体)的显着生化转化,由氧化角鲨烯环化酶(OSC)催化,构建了七个立体中心,使化学家着迷了半个多世纪。尽管已经报道了许多实验和理论上的努力,但是大多数研究集中在初始环化上,而随后的级联碳正离子重排和去质子化被忽略,这决定了与功能相关的结构域相关的最终产物羊毛甾醇的区域和立体化学胆固醇及其亲属。在此,我们继续对环我们以前的作品(Angew。化学。,诠释。埃德。2015年,54,8693–8696);通过量子力学/分子力学(QM / MM)分子动力学(MD)模拟研究了羊毛甾醇生物合成其余部分的机械细节,涉及三个氢化物转移,两个甲基转移和一个去质子化。我们获得了用于羊毛甾醇生物合成的完整自由能图谱,该动力学图谱在热力学和动力学上对于仅形成所需产物以及有效避免副产物是合理的。我们确定了一些关键因素,例如静电相互作用和CH··π相互作用,它们促进了碳正离子的迁移。我们还确定了直接去质子化的前体中间体I,这在以前的研究中一直引起争议。更重要的是,我们发现酶通过活性囊的电场介导了从初始环化到后续重排的能量转移,从而保证了酶催化的保真度。
更新日期:2020-01-23
中文翻译:

羊毛甾醇的生物合成机制:完整的故事
无环2,3-氧化角鲨烯到四环羊毛甾醇(胆固醇及其亲戚的常见前体)的显着生化转化,由氧化角鲨烯环化酶(OSC)催化,构建了七个立体中心,使化学家着迷了半个多世纪。尽管已经报道了许多实验和理论上的努力,但是大多数研究集中在初始环化上,而随后的级联碳正离子重排和去质子化被忽略,这决定了与功能相关的结构域相关的最终产物羊毛甾醇的区域和立体化学胆固醇及其亲属。在此,我们继续对环我们以前的作品(Angew。化学。,诠释。埃德。2015年,54,8693–8696);通过量子力学/分子力学(QM / MM)分子动力学(MD)模拟研究了羊毛甾醇生物合成其余部分的机械细节,涉及三个氢化物转移,两个甲基转移和一个去质子化。我们获得了用于羊毛甾醇生物合成的完整自由能图谱,该动力学图谱在热力学和动力学上对于仅形成所需产物以及有效避免副产物是合理的。我们确定了一些关键因素,例如静电相互作用和CH··π相互作用,它们促进了碳正离子的迁移。我们还确定了直接去质子化的前体中间体I,这在以前的研究中一直引起争议。更重要的是,我们发现酶通过活性囊的电场介导了从初始环化到后续重排的能量转移,从而保证了酶催化的保真度。

































 京公网安备 11010802027423号
京公网安备 11010802027423号