当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cl⋅⋅⋅Cl Halogen Bonding: Nature and Effect of Substituent at Electron Donor Cl atom
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-13 , DOI: 10.1002/slct.201903546
Dipankar Sutradhar 1 , Asit K. Chandra 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-13 , DOI: 10.1002/slct.201903546
Dipankar Sutradhar 1 , Asit K. Chandra 1
Affiliation
|
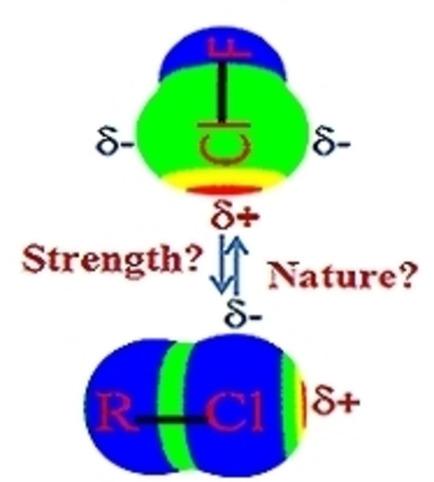
Halogen‐halogen bonding plays a vital role in crystal engineering and supramolecular chemistry. The Cl⋅⋅⋅Cl halogen bonding is studied here between R−Cl and ClF molecules using high level quantum chemical methods to find the nature and role of the substituent on the strength of this bond. A wide range of R moieties, like R=Cl, F, CH3, C2H5, C3H7, (CH3)3, H2F, CHF2, CF3, NH2, N(CH3)2, NHF and NF2, are chosen to find the effect of the substituent. The binding energies are found to range between −2.28 and −13.73 kJ mol−1 at the CCSD(T)/aug‐cc‐pVTZ level. The binding energy correlates with the negative electrostatic potential on the Cl atom and IP value of the R−Cl molecules, but it depends upon the nature of R−Cl bond. The electron density [ρ(rc)] at the bond critical point (BCP) is found to be a good parameter to assess the strength of the interaction. The decomposition of interaction energy by using Symmetry Adapted Perturbation Theory (SAPT) reveals that dispersion force is also important in stabilizing the complexes. Several important correlations between the strength of Cl⋅⋅⋅Cl interaction and molecular properties of R−Cl are established.
中文翻译:

Cl⋅⋅⋅Cl卤素键:电子给体Cl原子上取代基的性质和作用
卤素-卤素键在晶体工程和超分子化学中起着至关重要的作用。本文使用高级量子化学方法研究了RCl和ClF分子之间的Cl⋅⋅ClCl卤素键,以了解取代基的性质和作用对该键强度的作用。广泛的R部分,例如R = Cl,F,CH 3,C 2 H 5,C 3 H 7,(CH 3)3,H 2 F,CHF 2,CF 3,NH 2,N(CH 3)2,NHF和NF 2选择,以发现取代基的作用。发现在CCSD(T)/ aug-cc-pVTZ水平上,结合能在−2.28至−13.73 kJ mol -1之间。结合能与R-Cl分子的Cl原子上的负静电势和IP值相关,但这取决于R-Cl键的性质。发现键临界点(BCP)处的电子密度[ρ(r c)]是评估相互作用强度的良好参数。通过使用对称适应扰动理论(SAPT)分解相互作用能表明,分散力在稳定配合物中也很重要。建立了Cl⋅⋅⋅Cl相互作用强度与R-Cl分子性质之间的几个重要关系。
更新日期:2020-01-13
中文翻译:

Cl⋅⋅⋅Cl卤素键:电子给体Cl原子上取代基的性质和作用
卤素-卤素键在晶体工程和超分子化学中起着至关重要的作用。本文使用高级量子化学方法研究了RCl和ClF分子之间的Cl⋅⋅ClCl卤素键,以了解取代基的性质和作用对该键强度的作用。广泛的R部分,例如R = Cl,F,CH 3,C 2 H 5,C 3 H 7,(CH 3)3,H 2 F,CHF 2,CF 3,NH 2,N(CH 3)2,NHF和NF 2选择,以发现取代基的作用。发现在CCSD(T)/ aug-cc-pVTZ水平上,结合能在−2.28至−13.73 kJ mol -1之间。结合能与R-Cl分子的Cl原子上的负静电势和IP值相关,但这取决于R-Cl键的性质。发现键临界点(BCP)处的电子密度[ρ(r c)]是评估相互作用强度的良好参数。通过使用对称适应扰动理论(SAPT)分解相互作用能表明,分散力在稳定配合物中也很重要。建立了Cl⋅⋅⋅Cl相互作用强度与R-Cl分子性质之间的几个重要关系。

































 京公网安备 11010802027423号
京公网安备 11010802027423号