Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pluronic-F127/Platelet Microvesicles nanocomplex delivers stem cells in high doses to the bone marrow and confers post-irradiation survival.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-13 , DOI: 10.1038/s41598-019-57057-8
Vikas Chander 1 , Gurudutta Gangenahalli 1
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-13 , DOI: 10.1038/s41598-019-57057-8
Vikas Chander 1 , Gurudutta Gangenahalli 1
Affiliation
|
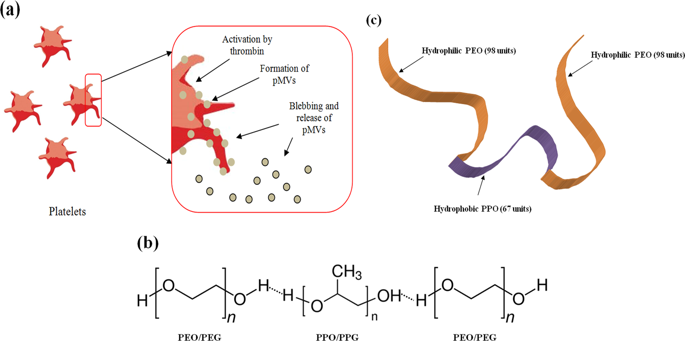
Platelet microvesicles (pMVs) are submicron-sized heterogeneous vesicles released upon activation and contain several membrane receptors and proteins (CD41, CD61, CD62, CXCR4, PAR-1, etc.). We have revealed their ability to adhere to the triblock copolymer pluronic-F127 (PF127) and form a platelet microvesicular nanocloud which has the potential to enhance the transvascular migration of hematopoietic stem cells across the sinusoidal endothelium to the bone marrow. Besides, the pMVs nanoclouds bestow survival benefits when present on the cells used for infusion, particularly with PF127-stabilized with chitosan-alginate (PF127-CA HSCs). The vesicles were found to be firmly associated with PF127 in the nanocloud, which was detected by confocal laser scanning microscopy. The abrogation of CXCR4/SDF-1 axis regulating the transmigration of the cells by antagonist AMD3100 revealed that the enriched CXCR4 receptors on pMVs robustize the transmigration of the infused cells. The homing of the cells led to effective engraftment and faster regeneration of the critical blood lineages, which elicited 100% survival of the mice receiving lethal doses of radiation. The Human Long-Term Culture Initiating Cells (LTC-ICs), Severe Combined Immunodeficient (SCID) - Repopulating Cells (SRCs) and Colony Forming Cells (CFCs) responsible for the regeneration, but present in extremely low numbers in the infused cell dose, have enabled the cells to reach the bone marrow in high numbers. This potential of the PF127 to sequester the pMVs and its application to achieve over 10-fold delivery of HSCs across the trans-endothelial checkpoint has so far not been reported. Thus, this mechanistic innovation is a potential post-exposure life-saving regimen capable of circumventing the irreparable damage to the bone marrow caused by lethal doses of radiation.
中文翻译:

Pluronic-F127/血小板微泡纳米复合物将高剂量的干细胞输送至骨髓并赋予辐射后存活率。
血小板微泡 (pMV) 是激活后释放的亚微米大小的异质囊泡,含有多种膜受体和蛋白质(CD41、CD61、CD62、CXCR4、PAR-1 等)。我们揭示了它们能够粘附到三嵌段共聚物普朗尼克-F127 (PF127) 上并形成血小板微泡纳米云,这有可能增强造血干细胞穿过血窦内皮向骨髓的跨血管迁移。此外,当 pMV 纳米云出现在用于输注的细胞上时,尤其是用壳聚糖-海藻酸盐稳定的 PF127(PF127-CA HSC)时,可带来生存益处。通过共焦激光扫描显微镜检测到,这些囊泡与纳米云中的 PF127 紧密相关。拮抗剂 AMD3100 废除了调节细胞迁移的 CXCR4/SDF-1 轴,表明 pMV 上富集的 CXCR4 受体增强了输注细胞的迁移。细胞的归巢导致关键血统的有效植入和更快的再生,从而使接受致命剂量辐射的小鼠 100% 存活。人类长期培养起始细胞 (LTC-IC)、严重联合免疫缺陷 (SCID) - 再生细胞 (SRC) 和集落形成细胞 (CFC) 负责再生,但在输注细胞剂量中的数量极少,使细胞能够大量到达骨髓。 PF127 隔离 pMV 的潜力及其在跨内皮检查点实现 10 倍以上 HSC 递送的应用迄今尚未见报道。 因此,这种机械创新是一种潜在的暴露后救生疗法,能够避免致命剂量的辐射对骨髓造成的不可挽回的损害。
更新日期:2020-01-13
中文翻译:

Pluronic-F127/血小板微泡纳米复合物将高剂量的干细胞输送至骨髓并赋予辐射后存活率。
血小板微泡 (pMV) 是激活后释放的亚微米大小的异质囊泡,含有多种膜受体和蛋白质(CD41、CD61、CD62、CXCR4、PAR-1 等)。我们揭示了它们能够粘附到三嵌段共聚物普朗尼克-F127 (PF127) 上并形成血小板微泡纳米云,这有可能增强造血干细胞穿过血窦内皮向骨髓的跨血管迁移。此外,当 pMV 纳米云出现在用于输注的细胞上时,尤其是用壳聚糖-海藻酸盐稳定的 PF127(PF127-CA HSC)时,可带来生存益处。通过共焦激光扫描显微镜检测到,这些囊泡与纳米云中的 PF127 紧密相关。拮抗剂 AMD3100 废除了调节细胞迁移的 CXCR4/SDF-1 轴,表明 pMV 上富集的 CXCR4 受体增强了输注细胞的迁移。细胞的归巢导致关键血统的有效植入和更快的再生,从而使接受致命剂量辐射的小鼠 100% 存活。人类长期培养起始细胞 (LTC-IC)、严重联合免疫缺陷 (SCID) - 再生细胞 (SRC) 和集落形成细胞 (CFC) 负责再生,但在输注细胞剂量中的数量极少,使细胞能够大量到达骨髓。 PF127 隔离 pMV 的潜力及其在跨内皮检查点实现 10 倍以上 HSC 递送的应用迄今尚未见报道。 因此,这种机械创新是一种潜在的暴露后救生疗法,能够避免致命剂量的辐射对骨髓造成的不可挽回的损害。

































 京公网安备 11010802027423号
京公网安备 11010802027423号