Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BCOR-coupled H2A monoubiquitination represses a subset of androgen receptor target genes regulating prostate cancer proliferation.
Oncogene ( IF 6.9 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41388-020-1153-3 Joanna K Lempiäinen 1 , A B M Kaiser Manjur 1 , Marjo Malinen 1, 2 , Kirsi Ketola 1 , Einari A Niskanen 1 , Jorma J Palvimo 1
Oncogene ( IF 6.9 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41388-020-1153-3 Joanna K Lempiäinen 1 , A B M Kaiser Manjur 1 , Marjo Malinen 1, 2 , Kirsi Ketola 1 , Einari A Niskanen 1 , Jorma J Palvimo 1
Affiliation
|
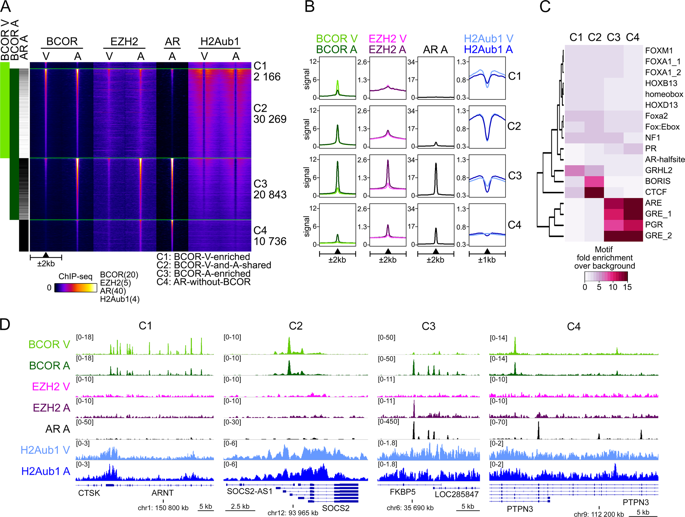
We have identified BCL6 corepressor (BCOR) as a hormone-dependent interaction partner of androgen receptor (AR), a key transcription factor in the development of normal and cancerous prostate. BCOR is often mutated in cancers and hematological diseases and as a component of a non-canonical polycomb repressive complex 1 (ncPRC1.1) required for arranging many facets of cellular differentiation. However, its role in androgen signaling or prostate cancer cells remains unknown. Here, our genome-wide analyses reveal that BCOR is recruited in an androgen-dependent fashion to majority of AR-binding chromatin sites in castration-resistant prostate cancer (CRPC) cells. Interestingly, depletion of BCOR has a significant effect on the expression of androgen-repressed genes linked to regulation of cell proliferation, differentiation and development. At many of these genes, such as HOX genes, the depletion leads to a decrease in H2A K119 monoubiquitination and an increase in mRNA expression. Consistently, BCOR depletion impairs the proliferation and viability of CRPC cells, inducing their apoptosis. Collectively, our data indicate a key role for the BCOR-ncPRC1.1 complex in the corepression of an important subset of AR target genes and the regulation of prostate cancer cell proliferation.
中文翻译:

BCOR偶联的H2A单泛素化抑制了调节前列腺癌增殖的雄激素受体靶基因的一个子集。
我们已经确定BCL6核心抑制剂(BCOR)是雄激素受体(AR)的激素依赖性相互作用伴侣,雄激素受体是正常和癌性前列腺发育中的关键转录因子。BCOR通常在癌症和血液疾病中发生突变,并且是安排细胞分化的许多方面所必需的非规范性多梳抑制复合物1(ncPRC1.1)的组成部分。然而,其在雄激素信号或前列腺癌细胞中的作用仍然未知。在这里,我们的全基因组分析表明,BCOR以雄激素依赖性方式募集到去势抵抗性前列腺癌(CRPC)细胞中大多数AR结合染色质位点。有趣的是,BCOR的消耗对雄激素抑制基因的表达具有重要影响,该基因与细胞增殖,分化和发育的调控有关。在许多此类基因(例如HOX基因)中,耗竭导致H2A K119单泛素化减少和mRNA表达增加。一致地,BCOR耗竭会损害CRPC细胞的增殖和活力,诱导其凋亡。总体而言,我们的数据表明BCOR-ncPRC1.1复合物在AR目标基因的重要子集的共抑制和前列腺癌细胞增殖的调控中起着关键作用。
更新日期:2020-01-10
中文翻译:

BCOR偶联的H2A单泛素化抑制了调节前列腺癌增殖的雄激素受体靶基因的一个子集。
我们已经确定BCL6核心抑制剂(BCOR)是雄激素受体(AR)的激素依赖性相互作用伴侣,雄激素受体是正常和癌性前列腺发育中的关键转录因子。BCOR通常在癌症和血液疾病中发生突变,并且是安排细胞分化的许多方面所必需的非规范性多梳抑制复合物1(ncPRC1.1)的组成部分。然而,其在雄激素信号或前列腺癌细胞中的作用仍然未知。在这里,我们的全基因组分析表明,BCOR以雄激素依赖性方式募集到去势抵抗性前列腺癌(CRPC)细胞中大多数AR结合染色质位点。有趣的是,BCOR的消耗对雄激素抑制基因的表达具有重要影响,该基因与细胞增殖,分化和发育的调控有关。在许多此类基因(例如HOX基因)中,耗竭导致H2A K119单泛素化减少和mRNA表达增加。一致地,BCOR耗竭会损害CRPC细胞的增殖和活力,诱导其凋亡。总体而言,我们的数据表明BCOR-ncPRC1.1复合物在AR目标基因的重要子集的共抑制和前列腺癌细胞增殖的调控中起着关键作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号