Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: an intricate interacting network including periplasmic and membrane proteins.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41598-019-56913-x Anne Bonneau 1, 2 , Béatrice Roche 1, 2 , Isabelle J Schalk 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41598-019-56913-x Anne Bonneau 1, 2 , Béatrice Roche 1, 2 , Isabelle J Schalk 1, 2
Affiliation
|
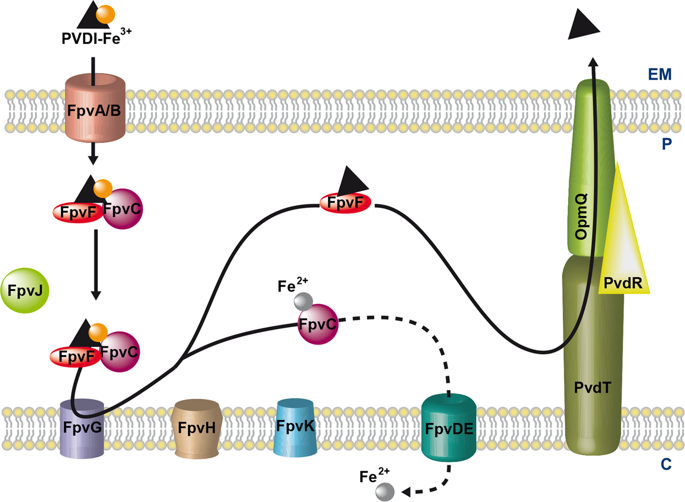
Pyoverdine (PVDI) has been reported to act both as a siderophore for scavenging iron (a key nutrient) and a signaling molecule for the expression of virulence factors. This compound is itself part of a core set of virulence factors produced by Pseudomonas aeruginosa during infections. Once secreted into the bacterial environment and having scavenged ferric iron, PVDI-Fe3+ is taken back into the P. aeruginosa periplasm via the outer membrane transporters FpvAI and FpvB. Iron release from PVDI in the bacterial periplasm involves numerous proteins encoded by the fpvGHJKCDEF genes and a mechanism of iron reduction. Here, we investigated the global interacting network between these various proteins using systematic bacterial two-hybrid screening. We deciphered a network of five interacting proteins composed of two inner-membrane proteins, FpvG (iron reductase) and FpvH (unknown function), and three periplasmic proteins, FpvJ (unknown function), FpvF (periplasmic PVDI-binding protein), and FpvC (iron periplasmic-binding protein). This interacting network strongly suggests the existence of a large protein machinery composed of these five proteins, all playing a role in iron acquisition by PVDI. Furthermore, we discovered an interaction between the periplasmic siderophore binding protein FpvF and the PvdRT-OpmQ efflux pump, also suggesting a role for FpvF in apo-PVDI recycling and secretion after iron delivery. These results highlight a multi-protein complex that drives iron release from PVDI in the periplasm of P. aeruginosa.
中文翻译:

铜铁吡over定在铜绿假单胞菌中获得铁:一个复杂的相互作用网络,包括周质和膜蛋白。
吡over定(PVDI)既可充当铁清除铁(一种关键营养素)的载体,又可充当表达毒力因子的信号分子。该化合物本身是铜绿假单胞菌在感染过程中产生的一组核心毒力因子的一部分。一旦被分泌到细菌环境中并清除了三价铁,PVDI-Fe3 +将通过外膜转运蛋白FpvAI和FpvB带回到铜绿假单胞菌周质中。细菌周质中PVDI中铁的释放涉及fpvGHJKCDEF基因编码的许多蛋白质以及铁还原的机制。在这里,我们使用系统的细菌双杂交筛选研究了这些蛋白质之间的全局相互作用网络。我们破译了由两个内膜蛋白组成的五个相互作用蛋白的网络,FpvG(铁还原酶)和FpvH(未知功能),以及三种周质蛋白,FpvJ(未知功能),FpvF(周质PVDI结合蛋白)和FpvC(铁周质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。和FpvC(铁质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。和FpvC(铁质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。我们发现周质铁载体结合蛋白FpvF和PvdRT-OpmQ外排泵之间存在相互作用,也表明FpvF在铁递送后apo-PVDI回收和分泌中发挥作用。这些结果突显了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。我们发现周质铁载体结合蛋白FpvF和PvdRT-OpmQ外排泵之间存在相互作用,也表明FpvF在铁递送后apo-PVDI回收和分泌中发挥作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。
更新日期:2020-01-10
中文翻译:

铜铁吡over定在铜绿假单胞菌中获得铁:一个复杂的相互作用网络,包括周质和膜蛋白。
吡over定(PVDI)既可充当铁清除铁(一种关键营养素)的载体,又可充当表达毒力因子的信号分子。该化合物本身是铜绿假单胞菌在感染过程中产生的一组核心毒力因子的一部分。一旦被分泌到细菌环境中并清除了三价铁,PVDI-Fe3 +将通过外膜转运蛋白FpvAI和FpvB带回到铜绿假单胞菌周质中。细菌周质中PVDI中铁的释放涉及fpvGHJKCDEF基因编码的许多蛋白质以及铁还原的机制。在这里,我们使用系统的细菌双杂交筛选研究了这些蛋白质之间的全局相互作用网络。我们破译了由两个内膜蛋白组成的五个相互作用蛋白的网络,FpvG(铁还原酶)和FpvH(未知功能),以及三种周质蛋白,FpvJ(未知功能),FpvF(周质PVDI结合蛋白)和FpvC(铁周质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。和FpvC(铁质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。和FpvC(铁质结合蛋白)。这个相互作用的网络强烈暗示了由这五个蛋白质组成的大型蛋白质机制的存在,它们在PVDI的铁捕获中均起着作用。此外,我们发现周质铁载体结合蛋白FpvF与PvdRT-OpmQ外排泵之间存在相互作用,也暗示FpvF在铁递送后apo-PVDI回收和分泌中的作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。我们发现周质铁载体结合蛋白FpvF和PvdRT-OpmQ外排泵之间存在相互作用,也表明FpvF在铁递送后apo-PVDI回收和分泌中发挥作用。这些结果突显了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。我们发现周质铁载体结合蛋白FpvF和PvdRT-OpmQ外排泵之间存在相互作用,也表明FpvF在铁递送后apo-PVDI回收和分泌中发挥作用。这些结果突出了一种多蛋白复合物,该复合物可驱动铜绿假单胞菌周质中的PVDI中的铁释放。































 京公网安备 11010802027423号
京公网安备 11010802027423号