当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring Interactions of Aptamers with Aβ40 Amyloid Aggregates and Its Application: Detection of Amyloid Aggregates.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.analchem.9b05493 Yan Zheng 1 , Xiuhua Geng 1 , Xiaohai Yang 1 , Shaoyuan Li 1 , Yaqin Liu 1 , Xiaofeng Liu 1 , Qing Wang 1 , Kemin Wang 1 , Ruichen Jia 1 , Yao Xu 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.analchem.9b05493 Yan Zheng 1 , Xiuhua Geng 1 , Xiaohai Yang 1 , Shaoyuan Li 1 , Yaqin Liu 1 , Xiaofeng Liu 1 , Qing Wang 1 , Kemin Wang 1 , Ruichen Jia 1 , Yao Xu 2
Affiliation
|
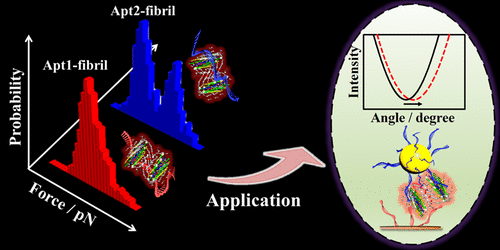
The exhaustive investigating interactions between recognition probes and amyloid aggregates, especially simultaneous recognition events, are challenging and crucial for the design of biosensing probes and further diagnosis of amyloid diseases. In the present work, the interactions of aptamers (Apts) with β-amyloid (Aβ) aggregates were explored thoroughly by single-molecule force spectroscopy (SMFS). Indeed, it was found that the interaction of aptamer1 (Apt1)-amyloid aggregates was different from that of aptamer2 (Apt2)-Aβ40 aggregates at the single-molecule level. Especially, the interaction force of Apt1-Aβ40 fibril showed a double distinguishing Gaussian fitting. The only unimodal distribution of the force histogram was displayed for the interactions of Apt2-Aβ40 oligomer, Apt2-Aβ40 fibril, and Apt1-Aβ40 oligomer. More intriguingly, two Apts could bind to amyloid aggregates simultaneously. With the assistance of two Apts recognition, a novel sensitive dual Apt-based surface plasmon resonance (SPR) sensor using Au nanoparticles (AuNPs) was developed for quantifying Aβ40 aggregates. The dual Apt-based SPR sensor not only avoided the limitation of steric hindrance and epitope but also employed simple operation as well as inexpensive recognition probes. A detection limit as low as 0.2 pM for Aβ40 oligomer and 0.05 pM for Aβ40 fibril could be achieved. Moreover, the established sensor could be successfully applied to detect Aβ40 aggregates in artificial cerebrospinal fluid (CSF) and undiluted real CSF. This work could provide a strategy to monitor a simultaneous recognition event using SMFS and broaden the application of Apts in the diagnosis of neurodegenerative diseases.
中文翻译:

探索适体与Aβ40淀粉样蛋白聚集体的相互作用及其应用:淀粉样蛋白聚集体的检测。
识别探针和淀粉样蛋白聚集体之间的详尽研究相互作用,尤其是同时发生的识别事件,对于生物传感探针的设计和淀粉样疾病的进一步诊断具有挑战性和至关重要的作用。在目前的工作中,通过单分子力谱(SMFS)彻底探讨了适体(Apts)与β-淀粉样蛋白(Aβ)聚集体的相互作用。确实,发现在单分子水平上,aptamer1(Apt1)-淀粉样聚集体的相互作用不同于aptamer2(Apt2)-Aβ40聚集体的相互作用。特别地,Apt1-Aβ40原纤维的相互作用力显示出双重区分的高斯拟合。力直方图的唯一单峰分布显示为Apt2-Aβ40低聚物,Apt2-Aβ40原纤维和Apt1-Aβ40低聚物之间的相互作用。更有趣的是 两个Apts可以同时结合淀粉样蛋白聚集体。借助两次Apts识别,开发了一种使用Au纳米颗粒(AuNPs)的新型敏感的基于双Apt的表面等离振子共振(SPR)传感器,用于定量Aβ40聚集体。基于双Apt的SPR传感器不仅避免了空间位阻和表位的限制,而且还采用了简单的操作以及廉价的识别探针。Aβ40低聚物的检出限低至0.2 pM,Aβ40原纤维的检出限低至0.05 pM。此外,所建立的传感器可以成功地用于检测人工脑脊液(CSF)和未稀释的真实CSF中的Aβ40聚集体。这项工作可以提供一种策略,以使用SMFS监视同时识别事件,并扩大Apts在神经退行性疾病诊断中的应用。
更新日期:2020-01-23
中文翻译:

探索适体与Aβ40淀粉样蛋白聚集体的相互作用及其应用:淀粉样蛋白聚集体的检测。
识别探针和淀粉样蛋白聚集体之间的详尽研究相互作用,尤其是同时发生的识别事件,对于生物传感探针的设计和淀粉样疾病的进一步诊断具有挑战性和至关重要的作用。在目前的工作中,通过单分子力谱(SMFS)彻底探讨了适体(Apts)与β-淀粉样蛋白(Aβ)聚集体的相互作用。确实,发现在单分子水平上,aptamer1(Apt1)-淀粉样聚集体的相互作用不同于aptamer2(Apt2)-Aβ40聚集体的相互作用。特别地,Apt1-Aβ40原纤维的相互作用力显示出双重区分的高斯拟合。力直方图的唯一单峰分布显示为Apt2-Aβ40低聚物,Apt2-Aβ40原纤维和Apt1-Aβ40低聚物之间的相互作用。更有趣的是 两个Apts可以同时结合淀粉样蛋白聚集体。借助两次Apts识别,开发了一种使用Au纳米颗粒(AuNPs)的新型敏感的基于双Apt的表面等离振子共振(SPR)传感器,用于定量Aβ40聚集体。基于双Apt的SPR传感器不仅避免了空间位阻和表位的限制,而且还采用了简单的操作以及廉价的识别探针。Aβ40低聚物的检出限低至0.2 pM,Aβ40原纤维的检出限低至0.05 pM。此外,所建立的传感器可以成功地用于检测人工脑脊液(CSF)和未稀释的真实CSF中的Aβ40聚集体。这项工作可以提供一种策略,以使用SMFS监视同时识别事件,并扩大Apts在神经退行性疾病诊断中的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号