当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pulse electrodeposition and corrosion properties of nanocrystalline nickel-chromium alloy coatings on copper substrate
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jallcom.2020.153712
Hossein Firouzi-Nerbin , Farzad Nasirpouri , Elnaz Moslehifard
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jallcom.2020.153712
Hossein Firouzi-Nerbin , Farzad Nasirpouri , Elnaz Moslehifard
|
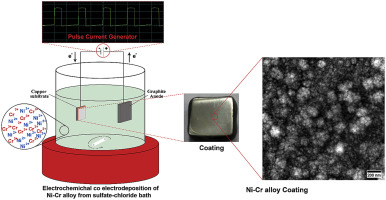
Abstract Co-electrodeposition of nickel-chromium (Ni–Cr) alloy coating was performed on a copper substrate from a sulfate-chloride bath. The effect of electrodeposition techniques namely direct (DC) and pulse-current (PC) techniques on cathodic efficiency, alloy composition, crystallite size, microhardness, morphology, and corrosion properties of Ni–Cr alloy coatings was investigated. Results show that PC electrodeposition generally produces coatings with desirable properties with respect to DC electrodeposition. Chemical composition measured by energy dispersive X-ray spectroscopy (EDS) and consequently other properties depend on PC parameters including peak current density and duty cycle whose increase rises Cr content of nickel-base alloy coatings. In contrast, PC frequency decreases the Cr content of Ni–Cr alloy coatings. When Cr content increases up to 24 wt% the crystallite size falls down to 66 nm. However, a phase segregation takes place by any further increase of Cr content than 24 wt% and therefore a new phase γˊ appears in the coatings. The surface morphology of the Ni–Cr coatings also changes from large spherical granules with a diameter ranging 27–114 nm to smaller size grain by increasing the Cr content. Above 24%wt. cracks appear on the coating surface. Based on potentiodynamic polarization method, Ni-11.2 wt % Cr alloy coating exhibits the highest corrosion resistance in 3.5 wt % NaCl compared to the other compositions. The corrosion resistance of the alloy coatings is mainly due to the formation of a passive film which in turn is enhanced by increasing the chromium content in the alloy. However, the corrosion resistance drops for high chromium Ni–Cr coatings (>24 wt %) owing to the microcracks developed in the coatings.
中文翻译:

铜基体纳米晶镍铬合金涂层的脉冲电沉积及腐蚀性能
摘要 镍铬 (Ni-Cr) 合金镀层在硫酸盐-氯化物浴中在铜基体上进行共电沉积。研究了电沉积技术,即直接 (DC) 和脉冲电流 (PC) 技术对 Ni-Cr 合金涂层阴极效率、合金成分、微晶尺寸、显微硬度、形貌和腐蚀性能的影响。结果表明,相对于直流电沉积,PC 电沉积通常会产生具有所需特性的涂层。通过能量色散 X 射线光谱 (EDS) 测量的化学成分以及其他性能取决于 PC 参数,包括峰值电流密度和占空比,其增加会提高镍基合金涂层的 Cr 含量。相比之下,PC 频率降低了 Ni-Cr 合金涂层的 Cr 含量。当 Cr 含量增加到 24 wt% 时,微晶尺寸下降到 66 nm。然而,当 Cr 含量超过 24 wt% 时,会发生相分离,因此涂层中会出现新的相 γˊ。通过增加 Cr 含量,Ni-Cr 涂层的表面形态也从直径为 27-114 nm 的大球形颗粒变为更小的颗粒。高于 24%wt。涂层表面出现裂纹。基于动电位极化方法,与其他组合物相比,Ni-11.2 wt % Cr 合金涂层在 3.5 wt % NaCl 中表现出最高的耐腐蚀性。合金涂层的耐腐蚀性能主要是由于钝化膜的形成,而钝化膜又通过增加合金中的铬含量而增强。然而,
更新日期:2020-05-01
中文翻译:

铜基体纳米晶镍铬合金涂层的脉冲电沉积及腐蚀性能
摘要 镍铬 (Ni-Cr) 合金镀层在硫酸盐-氯化物浴中在铜基体上进行共电沉积。研究了电沉积技术,即直接 (DC) 和脉冲电流 (PC) 技术对 Ni-Cr 合金涂层阴极效率、合金成分、微晶尺寸、显微硬度、形貌和腐蚀性能的影响。结果表明,相对于直流电沉积,PC 电沉积通常会产生具有所需特性的涂层。通过能量色散 X 射线光谱 (EDS) 测量的化学成分以及其他性能取决于 PC 参数,包括峰值电流密度和占空比,其增加会提高镍基合金涂层的 Cr 含量。相比之下,PC 频率降低了 Ni-Cr 合金涂层的 Cr 含量。当 Cr 含量增加到 24 wt% 时,微晶尺寸下降到 66 nm。然而,当 Cr 含量超过 24 wt% 时,会发生相分离,因此涂层中会出现新的相 γˊ。通过增加 Cr 含量,Ni-Cr 涂层的表面形态也从直径为 27-114 nm 的大球形颗粒变为更小的颗粒。高于 24%wt。涂层表面出现裂纹。基于动电位极化方法,与其他组合物相比,Ni-11.2 wt % Cr 合金涂层在 3.5 wt % NaCl 中表现出最高的耐腐蚀性。合金涂层的耐腐蚀性能主要是由于钝化膜的形成,而钝化膜又通过增加合金中的铬含量而增强。然而,

































 京公网安备 11010802027423号
京公网安备 11010802027423号