Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Novel Cell-Assisted Enhanced Chemiluminescence Strategy for Rapid and Label-Free Detection of Tumor Cells in Whole Blood.
ACS Sensors ( IF 8.2 ) Pub Date : 2020-01-16 , DOI: 10.1021/acssensors.9b02140 Lihua Ding 1 , Yongjun Wu 1 , Yanjuan Duan 1 , Songcheng Yu 1 , Fei Yu 1 , Jia Wang 1 , Yongmei Tian 1 , Zibo Gao 1 , Zhenzhen Wan 1 , Leiliang He 1
ACS Sensors ( IF 8.2 ) Pub Date : 2020-01-16 , DOI: 10.1021/acssensors.9b02140 Lihua Ding 1 , Yongjun Wu 1 , Yanjuan Duan 1 , Songcheng Yu 1 , Fei Yu 1 , Jia Wang 1 , Yongmei Tian 1 , Zibo Gao 1 , Zhenzhen Wan 1 , Leiliang He 1
Affiliation
|
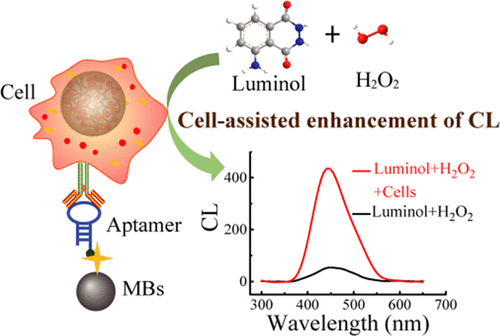
In this study, an interesting phenomenon was found where cells (including tumor and normal cells) managed to significantly enhance chemiluminescence (CL) signals. The possible reaction mechanism may be that cells can be severely damaged by CL substrates, and the released contents, possibly proteins (such as cytochrome c), can remarkably magnify CL owing to the increased production of singlet oxygen. More importantly, based on the above phenomena, a novel cell-assisted enhanced CL strategy was proposed for the rapid and label-free detection of tumor cells. The complexes of aptamer sgc8c and streptavidin-modified magnetic beads were employed to recognize and isolate target tumor cells from whole blood. The enhanced CL intensity, which was triggered directly by the captured cells, was measured. The proposed strategy exhibited a good detection performance with a linear range from 200 to 10,000 cells/mL. The analysis can be finished in ∼30 min, and the limit of detection was down to 100 cells/mL. The recoveries and relative standard deviations were 97.81-102.71% and 3.46-12.71%, respectively. Moreover, the established method can successfully distinguish the leukemia patients from healthy people. Therefore, it provides a novel, rapid, and simple method for the determination of tumor cells, which can be used in further practice.
中文翻译:

快速,无标记检测全血中肿瘤细胞的新型细胞辅助增强化学发光策略。
在这项研究中,发现了一个有趣的现象,其中细胞(包括肿瘤细胞和正常细胞)设法显着增强化学发光(CL)信号。可能的反应机制可能是细胞被CL底物严重破坏,并且由于单线态氧的产生增加,释放的内容物(可能是蛋白质(例如细胞色素c))可以显着放大CL。更重要的是,基于上述现象,提出了一种新型的细胞辅助增强CL策略,用于肿瘤细胞的快速和无标记检测。适体sgc8c和链霉亲和素修饰的磁珠的复合物用于识别和分离全血中的靶肿瘤细胞。测量了由捕获的细胞直接触发的增强的CL强度。所提出的策略表现出良好的检测性能,线性范围为200至10,000个细胞/ mL。分析可在约30分钟内完成,检测限降至100个细胞/ mL。回收率和相对标准偏差分别为97.81-102.71%和3.46-12.71%。而且,所建立的方法可以成功地将白血病患者与健康人区分开。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。建立的方法可以成功地区分白血病患者和健康人。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。建立的方法可以成功地区分白血病患者和健康人。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。
更新日期:2020-01-17
中文翻译:

快速,无标记检测全血中肿瘤细胞的新型细胞辅助增强化学发光策略。
在这项研究中,发现了一个有趣的现象,其中细胞(包括肿瘤细胞和正常细胞)设法显着增强化学发光(CL)信号。可能的反应机制可能是细胞被CL底物严重破坏,并且由于单线态氧的产生增加,释放的内容物(可能是蛋白质(例如细胞色素c))可以显着放大CL。更重要的是,基于上述现象,提出了一种新型的细胞辅助增强CL策略,用于肿瘤细胞的快速和无标记检测。适体sgc8c和链霉亲和素修饰的磁珠的复合物用于识别和分离全血中的靶肿瘤细胞。测量了由捕获的细胞直接触发的增强的CL强度。所提出的策略表现出良好的检测性能,线性范围为200至10,000个细胞/ mL。分析可在约30分钟内完成,检测限降至100个细胞/ mL。回收率和相对标准偏差分别为97.81-102.71%和3.46-12.71%。而且,所建立的方法可以成功地将白血病患者与健康人区分开。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。建立的方法可以成功地区分白血病患者和健康人。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。建立的方法可以成功地区分白血病患者和健康人。因此,它提供了一种新颖,快速,简单的确定肿瘤细胞的方法,可用于进一步的实践中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号