当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual functions of iridium(III) 2-phenylpyridine complexes: Metastasis inhibition and lysosomal damage.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.jinorgbio.2019.110983 Xicheng Liu 1 , Shujiao Chen 1 , Xingxing Ge 1 , Ying Zhang 1 , Yaoqi Xie 1 , Yingying Hao 1 , Daiqun Wu 1 , Jinmin Zhao 1 , Xiang-Ai Yuan 1 , Laijin Tian 1 , Zhe Liu 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.jinorgbio.2019.110983 Xicheng Liu 1 , Shujiao Chen 1 , Xingxing Ge 1 , Ying Zhang 1 , Yaoqi Xie 1 , Yingying Hao 1 , Daiqun Wu 1 , Jinmin Zhao 1 , Xiang-Ai Yuan 1 , Laijin Tian 1 , Zhe Liu 1
Affiliation
|
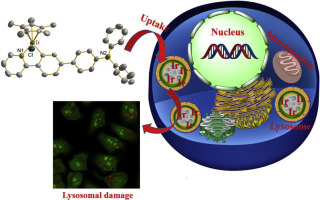
Six N-phenylcarbazole/triphenylamine-appended half-sandwich iridium(III) 2-phenylpyridine complexes ([(η5-Cp*)Ir(C^N)Cl]) were prepared and characterized. Compared with cisplatin, these complexes exhibited potential antitumor activity against A549 and HeLa tumor cells, with IC50 values (half-maximum inhibitory concentration) that changed from 2.8 ± 0.8 μM to 39.5 ± 2.7 μM, and could block the migration of tumor cells. These complexes also effectively bound to protein (binding constant: ~104 M-1) and were transported through serum proteins, catalyzed the oxidation of coenzyme nicotinamide-adenine dinucleotide. Additionally, laser confocal microscopy and flow cytometry confirmed that these complexes possessed a non-energy-dependent cellular uptake mechanism, effectively accumulated in lysosomes (Pearson colocalization coefficient: ~0.74), damaged the integrity of acidic lysosomes, led to a change in the mitochondrial membrane potential, disrupted the cell cycle (G0/G1 phase), and eventually induced apoptosis. Above all, these complexes are potential antitumor agents with dual functions: metastasis inhibition and lysosomal damage.
中文翻译:

铱(III)2-苯基吡啶配合物的双重功能:转移抑制和溶酶体破坏。
制备并表征了六个N-苯基咔唑/三苯胺加半夹心铱(III)2-苯基吡啶配合物([(η5-Cp*)Ir(C ^ N)Cl])。与顺铂相比,这些复合物对A549和HeLa肿瘤细胞具有潜在的抗肿瘤活性,IC50值(半数最大抑制浓度)从2.8±0.8μM变为39.5±2.7μM,并可能阻止肿瘤细胞的迁移。这些复合物还可以有效地与蛋白质结合(结合常数:〜104 M-1),并通过血清蛋白质转运,催化辅酶烟酰胺-腺嘌呤二核苷酸的氧化。此外,激光共聚焦显微镜和流式细胞术证实这些复合物具有非能量依赖性细胞摄取机制,有效地累积在溶酶体中(Pearson共定位系数:〜0.74),破坏酸性溶酶体的完整性,导致线粒体膜电位发生变化,破坏细胞周期(G0 / G1期),并最终诱导细胞凋亡。最重要的是,这些复合物是具有双重功能的潜在抗肿瘤药:转移抑制和溶酶体破坏。
更新日期:2020-01-08
中文翻译:

铱(III)2-苯基吡啶配合物的双重功能:转移抑制和溶酶体破坏。
制备并表征了六个N-苯基咔唑/三苯胺加半夹心铱(III)2-苯基吡啶配合物([(η5-Cp*)Ir(C ^ N)Cl])。与顺铂相比,这些复合物对A549和HeLa肿瘤细胞具有潜在的抗肿瘤活性,IC50值(半数最大抑制浓度)从2.8±0.8μM变为39.5±2.7μM,并可能阻止肿瘤细胞的迁移。这些复合物还可以有效地与蛋白质结合(结合常数:〜104 M-1),并通过血清蛋白质转运,催化辅酶烟酰胺-腺嘌呤二核苷酸的氧化。此外,激光共聚焦显微镜和流式细胞术证实这些复合物具有非能量依赖性细胞摄取机制,有效地累积在溶酶体中(Pearson共定位系数:〜0.74),破坏酸性溶酶体的完整性,导致线粒体膜电位发生变化,破坏细胞周期(G0 / G1期),并最终诱导细胞凋亡。最重要的是,这些复合物是具有双重功能的潜在抗肿瘤药:转移抑制和溶酶体破坏。































 京公网安备 11010802027423号
京公网安备 11010802027423号