当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C-H Insertion by Alkylidene Carbenes To Form 1,2,3-Triazines and Anionic [3 + 2] Dipolar Cycloadditions To Form Tetrazoles: Crucial Roles of Stereoelectronic and Steric Effects.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.orglett.9b04548 Fa-Jie Chen 1 , Yongjia Lin 2 , Man Xu 2 , Yuanzhi Xia 2 , Donald J Wink 1 , Daesung Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.orglett.9b04548 Fa-Jie Chen 1 , Yongjia Lin 2 , Man Xu 2 , Yuanzhi Xia 2 , Donald J Wink 1 , Daesung Lee 1
Affiliation
|
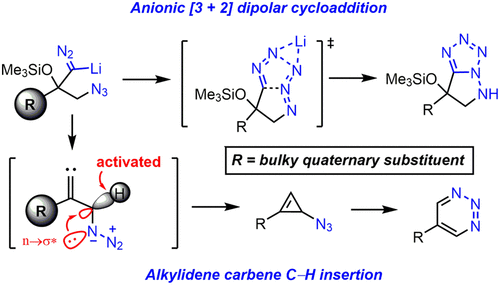
The synthesis of 1,2,3-triazines and bicyclic tetrazoles from α-azido ketones is described. The common intermediate generated from lithiated trimethylsilyldiazomethane and α-azido ketones diverges depending on the steric bulk of the substituents. The formation of 1,2,3-triazines via a C-H insertion of alkylidene carbene to form 3-azidocyclopropene, followed by its rearrangement, is supported by density functional theory calculations. Tetrazole formation proceeds via a facile anionic [3 + 2] dipolar cycloaddition between a lithiated diazo moiety and an azido group facilitated by the chelation of a lithium ion.
中文翻译:

通过亚烷基卡宾苯生成1,2,3-三嗪和形成[3 + 2]阴离子偶极环加成反应生成四唑的CH插入:立体电子和立体效应的关键作用。
描述了由α-叠氮基酮合成1,2,3-三嗪和双环四唑。由锂化的三甲基甲硅烷基重氮甲烷和α-叠氮基酮生成的常见中间体根据取代基的空间体积而不同。密度泛函理论计算支持通过亚烷基卡宾的CH插入形成3-叠氮环丙烯,然后进行重排而形成1,2,3-三嗪。通过锂化螯合促进的锂化重氮基团和叠氮基之间的阴离子[3 + 2]偶极环加成反应,可形成四唑。
更新日期:2020-01-07
中文翻译:

通过亚烷基卡宾苯生成1,2,3-三嗪和形成[3 + 2]阴离子偶极环加成反应生成四唑的CH插入:立体电子和立体效应的关键作用。
描述了由α-叠氮基酮合成1,2,3-三嗪和双环四唑。由锂化的三甲基甲硅烷基重氮甲烷和α-叠氮基酮生成的常见中间体根据取代基的空间体积而不同。密度泛函理论计算支持通过亚烷基卡宾的CH插入形成3-叠氮环丙烯,然后进行重排而形成1,2,3-三嗪。通过锂化螯合促进的锂化重氮基团和叠氮基之间的阴离子[3 + 2]偶极环加成反应,可形成四唑。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号