当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Deoxypseudouridine 5'-Triphosphate Bearing the Photoremovable Protecting Group at the N1 Position Capable of Enzymatic Incorporation to DNA.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.joc.9b02194 Leo Takeshita 1 , Yuji Yamada 1 , Yoshiaki Masaki 1 , Kohji Seio 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acs.joc.9b02194 Leo Takeshita 1 , Yuji Yamada 1 , Yoshiaki Masaki 1 , Kohji Seio 1
Affiliation
|
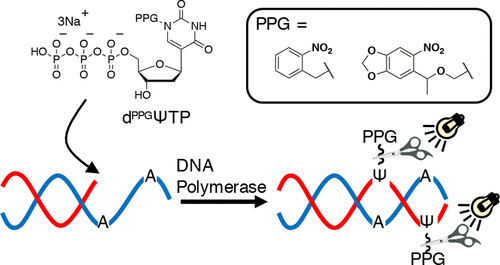
Enzymatic incorporation of deoxynucleoside 5'-triphosphate bearing the photocleavable protecting group is a useful method for the preparation of photocaged oligodeoxynucleotides. Here, we describe the synthesis of new photocaged deoxynucleoside triphosphates N1-(2-nitrobenzyl)-deoxypseudouridine triphosphate (dNBΨTP) and N1-(6-nitropiperonyloxymethyl)-deoxypseudouridine triphosphate (dNPOMΨTP). We successfully synthesized dNBΨTP and dNPOMΨTP and applied them to enzymatic synthesis of photocaged oligonucleotides. In addition, we also synthesized phosphoramidites of N1-(2-nitrobenzyl)- and N1-(6-nitropiperonyloxymethyl)-deoxypseudouridine to enable chemical synthesis of photocaged oligonucleotides incorporating them. The photocleavable 2-nitrobenzyl and 6-nitropiperonyloxymethyl in oligonucleotides were cleaved by irradiation at 365 nm for 30 and 10 s, respectively. We also studied the enzymatic incorporation of dNBΨTP and dNPOMΨTP using the Klenow fragment exo-. As a result, it was clarified that dNPOMΨTP could be incorporated to oligonucleotide 193 times more efficiently than dNBΨTP, as judged by Vmax/Km. We also performed the incorporation of at least eight dNPOMΨ residues in a 35-mer oligodeoxynucleotide. It has also been revealed that the oligodeoxynucleotides incorporating photocaged deoxypseudouridine were useful for photocontrol of DNA triplex formation.
中文翻译:

能够在N1位置酶解掺入DNA的带有可光去除保护基团的脱氧伪神经杜啶5'-三磷酸酯的合成。
带有光可裂解保护基的脱氧核苷5'-三磷酸的酶促掺入是制备光笼化的寡脱氧核苷酸的有用方法。在这里,我们描述了新的光笼式脱氧核苷三磷酸酯N1-(2-硝基苄基)-脱氧pseudouridine三磷酸(dNBΨTP)和N1-(6-硝基胡椒基氧基甲基)-脱氧pseudouridine三磷酸(dNPOMΨTP)的合成。我们成功地合成了dNBΨTP和dNPOMΨTP,并将其应用于光笼寡核苷酸的酶促合成。另外,我们还合成了N1-(2-硝基苄基)-和N1-(6-硝基哌啶基氧基甲基)-脱氧伪杜鹃碱的亚磷酰胺,以使掺入它们的光笼寡核苷酸能够化学合成。通过在365nm下照射30秒和10s分别裂解寡核苷酸中的可光裂解的2-硝基苄基和6-硝基哌啶基氧基甲基。我们还研究了使用Klenow片段exo-酶促整合dNBΨTP和dNPOMΨTP的方法。结果表明,根据Vmax / Km判断,dNPOMΨTP可以比dNBΨTP高193倍地掺入寡核苷酸。我们还进行了一个35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。根据Vmax / Km的判断,已证实dNPOMΨTP掺入寡核苷酸的效率是dNBΨTP的193倍。我们还进行了35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。根据Vmax / Km的判断,已证实dNPOMΨTP掺入寡核苷酸的效率是dNBΨTP的193倍。我们还进行了一个35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。
更新日期:2020-01-07
中文翻译:

能够在N1位置酶解掺入DNA的带有可光去除保护基团的脱氧伪神经杜啶5'-三磷酸酯的合成。
带有光可裂解保护基的脱氧核苷5'-三磷酸的酶促掺入是制备光笼化的寡脱氧核苷酸的有用方法。在这里,我们描述了新的光笼式脱氧核苷三磷酸酯N1-(2-硝基苄基)-脱氧pseudouridine三磷酸(dNBΨTP)和N1-(6-硝基胡椒基氧基甲基)-脱氧pseudouridine三磷酸(dNPOMΨTP)的合成。我们成功地合成了dNBΨTP和dNPOMΨTP,并将其应用于光笼寡核苷酸的酶促合成。另外,我们还合成了N1-(2-硝基苄基)-和N1-(6-硝基哌啶基氧基甲基)-脱氧伪杜鹃碱的亚磷酰胺,以使掺入它们的光笼寡核苷酸能够化学合成。通过在365nm下照射30秒和10s分别裂解寡核苷酸中的可光裂解的2-硝基苄基和6-硝基哌啶基氧基甲基。我们还研究了使用Klenow片段exo-酶促整合dNBΨTP和dNPOMΨTP的方法。结果表明,根据Vmax / Km判断,dNPOMΨTP可以比dNBΨTP高193倍地掺入寡核苷酸。我们还进行了一个35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。根据Vmax / Km的判断,已证实dNPOMΨTP掺入寡核苷酸的效率是dNBΨTP的193倍。我们还进行了35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。根据Vmax / Km的判断,已证实dNPOMΨTP掺入寡核苷酸的效率是dNBΨTP的193倍。我们还进行了一个35聚体寡脱氧核苷酸中至少八个dNPOM 3残基的掺入。还已经揭示了掺入光笼式脱氧伪神经杜里定的寡脱氧核苷酸可用于光控制DNA三链体的形成。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号