Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-01-07 , DOI: 10.1016/j.molliq.2019.112429 Ankita Ravani , Atindra Shukla , Nandhibatla Vishwanada Sastry , Dinesh Ochhavlal Shah , Manish Kumar Mishra
|
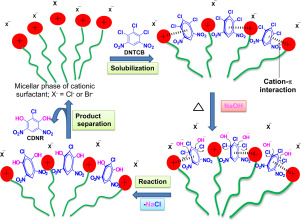
The micellar catalysis of the hydroxylation of 1,2,3-trichloro-4,6-dinitrobenzene (DNTCB) with aqueous NaOH, to synthesize 2-chloro-4,6-dinitroresorcinol (CDNR), was studied using cationic, anionic and non-ionic surfactants at different concentrations to promote the reaction kinetics. The micellar catalysis using cationic surfactants at optimum concentrations was effective giving highest conversion of DNTCB (92–100%), which was ascribed to high solubilization capacity of the micellar solutions for DNTCB, surface positive charges of micelles increasing OH– ions concentration near micellar surface, and the cation-π interaction of surfactant molecules (through cationic head group) with DNTCB molecules. The cation-π interaction plays important role in solubilization process, substrate activation and catalysis of the reaction. The higher surfactant concentrations retard the reaction owing to strong solubilization of DNTCB in micelles and reduced OH– ions concentration around the micelles. The cationic micellar solutions at optimum surfactant concentrations can be effective catalytic system for the aromatic nucleophilic substitution reactions.
中文翻译:

胶束催化的1,2,3-三氯-4,6-二硝基苯的羟基化:阳离子头基-π相互作用的作用
用阳离子,阴离子和非离子水研究了胶束催化1,2,3-三氯-4,6-二硝基苯(DNTCB)在NaOH水溶液中的羟基化反应,合成2-氯-4,6-二硝基间苯二酚(CDNR)。离子型表面活性剂以不同的浓度促进反应动力学。使用最佳浓度的阳离子表面活性剂进行胶束催化可有效实现DNTCB的最高转化率(92–100%),这归因于胶束溶液对DNTCB的高溶解能力,胶束的表面正电荷增加了OH –胶束表面附近的离子浓度,以及表面活性剂分子(通过阳离子头基)与DNTCB分子的阳离子-π相互作用。阳离子-π相互作用在增溶过程,底物活化和反应催化中起重要作用。较高的表面活性剂浓度延缓由于DNTCB的强溶解在胶束中的反应和减少的OH -离子浓度周围的胶束。阳离子胶束溶液在最佳表面活性剂浓度下可以成为芳族亲核取代反应的有效催化体系。






























 京公网安备 11010802027423号
京公网安备 11010802027423号