Nature Catalysis ( IF 42.8 ) Pub Date : 2020-01-06 , DOI: 10.1038/s41929-019-0386-4
Yanwei Lum , Jianan Erick Huang , Ziyun Wang , Mingchuan Luo , Dae-Hyun Nam , Wan Ru Leow , Bin Chen , Joshua Wicks , Yuguang C. Li , Yuhang Wang , Cao-Thang Dinh , Jun Li , Tao-Tao Zhuang , Fengwang Li , Tsun-Kong Sham , David Sinton , Edward H. Sargent
|
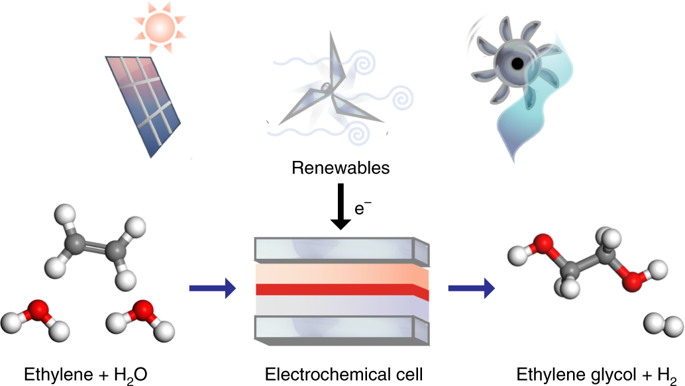
There is significant interest in developing efficient electrochemical processes for commodity chemical manufacturing, all directly powered by renewable electricity. A vital chemical is ethylene glycol, with an annual consumption of around 20 million tonnes due to its use as antifreeze and as a polymer precursor. Here we report a one-step electrochemical route at ambient temperature and pressure in aqueous media to the selective partial oxidation of ethylene to ethylene glycol. Tuning of the catalyst OH binding energy was hypothesized to be crucial for facilitating the transfer of OH to *C2H4OH to form ethylene glycol. Computational studies suggested that a gold-doped palladium catalyst could perform this step efficiently, and experimentally we found it to exhibit an approximate 80% Faradaic efficiency to ethylene glycol, retaining its performance for 100 hours of continuous operation. These findings represent a significant advance in the development of selective anodic partial oxidation reactions in aqueous media under mild conditions.
中文翻译:

调节OH结合能可实现乙烯选择性电化学氧化为乙二醇
开发用于商品化学制造的有效电化学方法的兴趣浓厚,这些工艺均直接由可再生电力提供动力。至关重要的化学品是乙二醇,由于用作防冻剂和聚合物前体,因此每年的消费量约为2000万吨。在这里,我们报告了一种在环境温度和压力下在水性介质中将乙烯选择性部分氧化为乙二醇的一步式电化学路线。催化剂的OH结合能的调整被认为对于促进OH向* C 2 H 4的转移至关重要。OH形成乙二醇。计算研究表明,掺金的钯催化剂可以有效地执行此步骤,并且通过实验我们发现它对乙二醇的法拉第效率约为80%,并在连续运行100小时后仍能保持其性能。这些发现代表了在温和条件下在水性介质中选择性阳极部分氧化反应发展的重大进展。

































 京公网安备 11010802027423号
京公网安备 11010802027423号