当前位置:
X-MOL 学术
›
JACC Cardiovasc. Inte.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Randomized Comparison of Ridaforolimus-Eluting and Zotarolimus-Eluting Coronary Stents: 2-Year Clinical Outcomes From the BIONICS and NIREUS Trials.
JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jcin.2019.08.019
Maayan Konigstein 1 , Pieter C Smits 2 , Michael P Love 3 , Ori Ben-Yehuda 4 , Melek Ozgu Ozan 5 , Mengdan Liu 5 , Gidon Y Perlman 6 , Martin B Leon 4 , Gregg W Stone 5 , David E Kandzari 7
JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jcin.2019.08.019
Maayan Konigstein 1 , Pieter C Smits 2 , Michael P Love 3 , Ori Ben-Yehuda 4 , Melek Ozgu Ozan 5 , Mengdan Liu 5 , Gidon Y Perlman 6 , Martin B Leon 4 , Gregg W Stone 5 , David E Kandzari 7
Affiliation
|
OBJECTIVES
This study sought to determine clinical outcomes between treatment groups over long-term follow-up.
BACKGROUND
The safety and efficacy of a ridaforolimus-eluting stent (RES) was evaluated in the BIONICS (BioNIR Ridaforolimus-Eluting Coronary Stent System in Coronary Stenosis) and NIREUS (BioNIR Ridaforolimus Eluting Coronary Stent System [BioNIR] European Angiography Study) trials, demonstrating noninferiority of RES in comparison with a zotarolimus-eluting stent (ZES) regarding 1-year target lesion failure (TLF) and 6-month angiographic late lumen loss, respectively.
METHODS
Patient-level data from the BIONICS (N = 1,919) and NIREUS (N = 302) randomized trials were pooled, and outcomes in patients implanted with RES and ZES compared. Broad inclusion criteria allowed enrollment of patients with acute coronary syndromes and complex lesions. The primary endpoint was the 2-year rate of TLF or clinically driven target lesion revascularization.
RESULTS
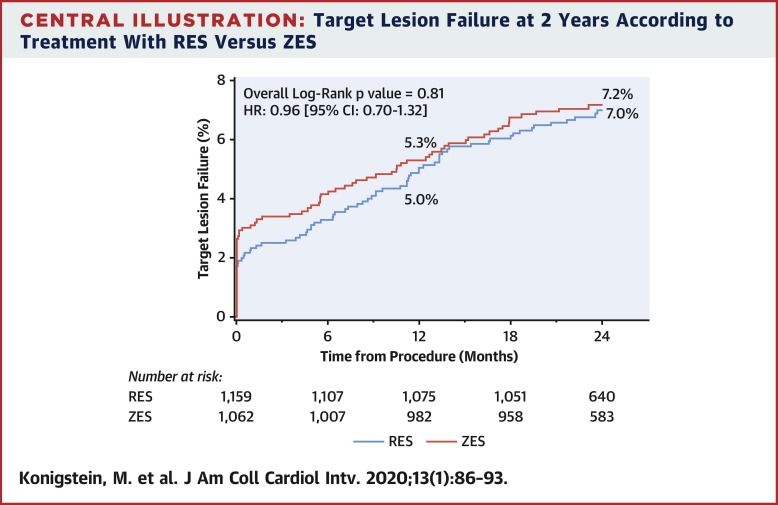
A total of 2,221 patients (age 63.2 ± 10.3 years; 79.7% men) undergoing percutaneous coronary intervention with RES (n = 1,159) or ZES (n = 1,062) were included. Clinical and angiographic characteristics were similar between groups. At 2 years, the primary endpoint of TLF was similar among patients implanted with RES and ZES (7.0% vs. 7.2%; p = 0.94). Rates of target lesion revascularization (4.8% RES vs. 4.1% ZES; p = 0.41) and target vessel-related myocardial infarction (3.1% RES vs. 3.8% ZES; p = 0.52) did not differ between groups. The overall rate of stent thrombosis was also similar (0.5% RES vs. 0.9% ZES; p = 0.39).
CONCLUSIONS
In a pooled analysis of 2 randomized trials, 2-year clinical outcomes were similar between patients undergoing percutaneous coronary intervention with RES and ZES. These results support the long-term safety and efficacy of RES for the treatment of a broad population of patients with coronary artery disease.
中文翻译:

Ridaforolimus洗脱和Zotarolimus洗脱冠状动脉支架的随机比较:BIONICS和NIREUS试验的2年临床结果。
目的本研究旨在确定长期随访后各治疗组之间的临床结局。背景技术在BIONICS(BioNIR冠状动脉狭窄中的BioNIR Ridaforolimus洗脱冠状动脉支架系统)和NIREUS(BioNIR Ridaforolimus洗脱冠状动脉支架系统[BioNIR]欧洲血管造影研究)试验中评估了ridaforolimus洗脱支架(RES)的安全性和有效性。与1年目标病变失败(TLF)和6个月血管造影晚期管腔丢失有关的RES与zotarolimus洗脱支架(ZES)相比的非劣效性。方法收集来自BIONICS(N = 1,919)和NIREUS(N = 302)随机试验的患者水平数据,并比较植入RES和ZES的患者的结局。广泛的纳入标准允许急性冠脉综合征和复杂病变的患者入组。主要终点是TLF或临床驱动的靶病变血运重建的2年率。结果总共纳入了2221例接受RES(n = 1,159)或ZES(n = 1,062)的经皮冠状动脉介入治疗的患者(63.2±10.3岁;男性为79.7%)。两组之间的临床和血管造影特征相似。在2年时,植入RES和ZES的患者中TLF的主要终点相似(分别为7.0%和7.2%; p = 0.94)。两组之间的目标病变血运重建率(4.8%RES vs. 4.1%ZES; p = 0.41)和目标血管相关的心肌梗塞(3.1%RES vs. 3.8%ZES; p = 0.52)没有差异。支架血栓形成的总发生率也相似(0.5%RES比0.9%ZES; p = 0.39)。结论在对2项随机试验的汇总分析中,接受RES和ZES经皮冠状动脉介入治疗的患者的2年临床结果相似。这些结果支持RES用于治疗大量冠心病患者的长期安全性和有效性。
更新日期:2020-01-07
中文翻译:

Ridaforolimus洗脱和Zotarolimus洗脱冠状动脉支架的随机比较:BIONICS和NIREUS试验的2年临床结果。
目的本研究旨在确定长期随访后各治疗组之间的临床结局。背景技术在BIONICS(BioNIR冠状动脉狭窄中的BioNIR Ridaforolimus洗脱冠状动脉支架系统)和NIREUS(BioNIR Ridaforolimus洗脱冠状动脉支架系统[BioNIR]欧洲血管造影研究)试验中评估了ridaforolimus洗脱支架(RES)的安全性和有效性。与1年目标病变失败(TLF)和6个月血管造影晚期管腔丢失有关的RES与zotarolimus洗脱支架(ZES)相比的非劣效性。方法收集来自BIONICS(N = 1,919)和NIREUS(N = 302)随机试验的患者水平数据,并比较植入RES和ZES的患者的结局。广泛的纳入标准允许急性冠脉综合征和复杂病变的患者入组。主要终点是TLF或临床驱动的靶病变血运重建的2年率。结果总共纳入了2221例接受RES(n = 1,159)或ZES(n = 1,062)的经皮冠状动脉介入治疗的患者(63.2±10.3岁;男性为79.7%)。两组之间的临床和血管造影特征相似。在2年时,植入RES和ZES的患者中TLF的主要终点相似(分别为7.0%和7.2%; p = 0.94)。两组之间的目标病变血运重建率(4.8%RES vs. 4.1%ZES; p = 0.41)和目标血管相关的心肌梗塞(3.1%RES vs. 3.8%ZES; p = 0.52)没有差异。支架血栓形成的总发生率也相似(0.5%RES比0.9%ZES; p = 0.39)。结论在对2项随机试验的汇总分析中,接受RES和ZES经皮冠状动脉介入治疗的患者的2年临床结果相似。这些结果支持RES用于治疗大量冠心病患者的长期安全性和有效性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号