当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific Synthesis of Nepetalactones by One-Step Oxidative NHC Catalysis.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.orglett.9b04034
Wacharee Harnying 1 , Jörg-M Neudörfl 1 , Albrecht Berkessel 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.orglett.9b04034
Wacharee Harnying 1 , Jörg-M Neudörfl 1 , Albrecht Berkessel 1
Affiliation
|
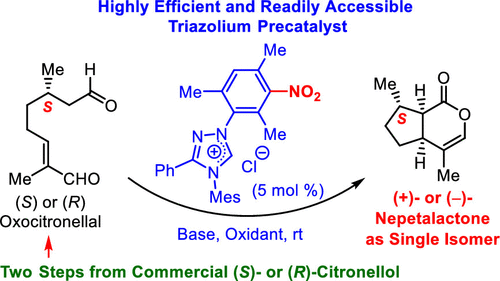
An efficient oxidative NHC-catalyzed one-step transformation of (S)- or (R)-8-oxocitronellal to nepetalactone (NL) in enantio- and diastereomerically pure form has been developed. Several new and "easy to make" N-Mes- or N-Dipp-substituted 1,2,4-triazolium salts carrying nitroaromatic groups on N1 were synthesized and evaluated as precatalysts in combination with base and stoichiometric organic oxidant. Under optimized conditions, NLs are accessible in very good yields and diastereomerically pure under mild conditions. The oxidant used could be recovered and recycled under operationally simple conditions.
中文翻译:

通过一步氧化NHC催化对映体合成荆芥内酯。
已经开发了一种有效的氧化NHC催化的对映体和非对映体纯形式的(S)-或(R)-8-氧鞘甾醇向荆芥内酯(NL)的一步转化。合成了几种在N1上带有硝基芳族基团的新型且易于制造的N-Mes或N-Dipp取代的1,2,4-三唑鎓盐,并与碱和化学计量有机氧化剂一起作为预催化剂进行了评估。在优化的条件下,NLs的收率非常好,在温和条件下非对映异构纯。所使用的氧化剂可在操作简单的条件下回收和再循环。
更新日期:2020-01-06
中文翻译:

通过一步氧化NHC催化对映体合成荆芥内酯。
已经开发了一种有效的氧化NHC催化的对映体和非对映体纯形式的(S)-或(R)-8-氧鞘甾醇向荆芥内酯(NL)的一步转化。合成了几种在N1上带有硝基芳族基团的新型且易于制造的N-Mes或N-Dipp取代的1,2,4-三唑鎓盐,并与碱和化学计量有机氧化剂一起作为预催化剂进行了评估。在优化的条件下,NLs的收率非常好,在温和条件下非对映异构纯。所使用的氧化剂可在操作简单的条件下回收和再循环。

































 京公网安备 11010802027423号
京公网安备 11010802027423号