Chem ( IF 19.1 ) Pub Date : 2020-01-06 , DOI: 10.1016/j.chempr.2019.12.010 Yadong Gao , Chao Yang , Songlin Bai , Xiaolei Liu , Qingcui Wu , Jing Wang , Chao Jiang , Xiangbing Qi
|
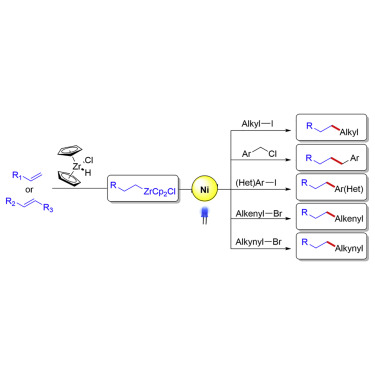
Transition-metal-catalyzed cross-coupling reactions between naturally abundant sp3-hybridized carbon centers facilitate access to diverse molecules with complex three-dimensional structures. Organometallic compounds are among one of the most powerful reagents that are broadly used in carbon–carbon bond formations. Although sp2-hybridized organometallic compounds are widely employed in cross-couplings, sp3-hybridized organometallic coupling partners are less developed. Herein, we report visible-light-induced single nickel-catalyzed C(sp3)–C(sp3), C(sp3)–C(sp2), and C(sp3)–C(sp) cross-coupling reactions using alkylzirconocenes, which are easily generated in situ from terminal or internal unactivated alkenes through hydrozirconation and chain walking. This method is mild and applicable for a large range of substrates including primary, secondary, tertiary alkyl, aryl, alkenyl, alkynyl halides, and a variety of alkenes. Mechanistic studies suggest a novel nickel-catalyzed radical cross-coupling pathway, which represents the first visible-light-induced transformation of alkylzirconocenes.
中文翻译:

可见光诱导的镍催化与未活化烯烃的烷基锆茂新星交叉偶联。
自然丰富的sp 3-杂化碳中心之间的过渡金属催化交叉偶联反应有助于获得具有复杂三维结构的各种分子。有机金属化合物是最强大的试剂之一,广泛用于碳-碳键的形成。尽管sp 2-杂化的有机金属化合物广泛用于交叉偶联,但是sp 3-杂化的有机金属偶联剂的开发较少。在这里,我们报告可见光诱导的单个镍催化的C(sp 3)–C(sp 3),C(sp 3)–C(sp 2)和C(sp 3)–C(sp)使用烷基锆茂的交叉偶联反应,该反应很容易从末端或内部未活化的烯烃通过加氢锆和链步反应就地生成。该方法温和,适用于多种底物,包括伯,仲,叔烷基,芳基,烯基,炔基卤化物和各种烯烃。机理研究表明,镍催化的自由基交叉偶联是一种新颖的途径,代表了锆锆茂的首次可见光诱导的转变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号