当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Structural Evolution and Redox Mechanism of a NaFeO2–NaCoO2 Solid Solution for Sodium‐Ion Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2016-06-23 , DOI: 10.1002/adfm.201601292
Kei Kubota 1, 2 , Takuya Asari 1 , Hiroaki Yoshida 1 , Naoaki Yaabuuchi 1, 2 , Hiromasa Shiiba 3 , Masanobu Nakayama 2, 3 , Shinichi Komaba 1, 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2016-06-23 , DOI: 10.1002/adfm.201601292
Kei Kubota 1, 2 , Takuya Asari 1 , Hiroaki Yoshida 1 , Naoaki Yaabuuchi 1, 2 , Hiromasa Shiiba 3 , Masanobu Nakayama 2, 3 , Shinichi Komaba 1, 2
Affiliation
|
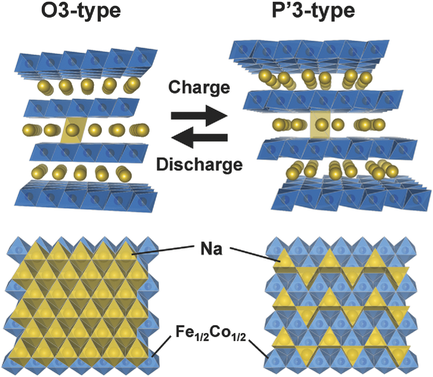
Na‐ion batteries have become promising candidates for large‐scale energy‐storage systems because of the abundant Na resources and they have attracted considerable academic interest because of their unique behavior, such as their electrochemical activity for the Fe3+/Fe4+ redox couple. The high‐rate performance derived from the low Lewis‐acidity of the Na+ ions is another advantage of Na‐ion batteries and has been demonstrated in NaFe1/2Co1/2O2 solutions. Here, a solid solution of NaFeO2‐NaCoO2 is synthesized and the mechanisms behind their excellent electrochemical performance are studied in comparison to those of their respective end‐members. The combined analysis of operando X‐ray diffraction, ex situ X‐ray absorption spectroscopy, and density functional theory (DFT) calculations for Na1–
x
Fe1/2Co1/2O2 reveals that the O3‐type phase transforms into a P3‐type phase coupled with Na+/vacancy ordering, which has not been observed in O3‐type NaFeO2. The substitution of Co for Fe stabilizes the P3‐type phase formed by sodium extraction and could suppress the irreversible structural change that is usually observed in O3‐type NaFeO2, resulting in a better cycle retention and higher rate performance. Although no ordering of the transition metal ions is seen in the neutron diffraction experiments, as supported by Monte‐Carlo simulations, the formation of a superlattice originating from the Na+/vacancy ordering is found by synchrotron X‐ray diffraction for Na0.5Fe1/2Co1/2O2, which may involve a potential step in the charge/discharge profiles.
中文翻译:

了解钠离子电池用NaFeO2–NaCoO2固溶体的结构演变和氧化还原机理
Na离子电池由于其丰富的Na资源而已成为大规模储能系统的有希望的候选者,并且由于其独特的行为(例如其对Fe 3+ / Fe 4+的氧化还原的电化学活性)已经引起了相当大的学术兴趣。夫妻。Na +离子的低路易斯酸度带来的高效率性能是Na-离子电池的另一个优势,并且已在NaFe 1/2 Co 1/2 O 2溶液中得到证明。这里是NaFeO 2 -NaCoO 2的固溶体合成了它们,并与它们各自的末端成员进行了比较,研究了其出色的电化学性能背后的机理。对Na 1– x Fe 1/2 Co 1/2 O 2的操作X射线衍射,非原位X射线吸收光谱和密度泛函理论(DFT)计算的综合分析显示,O3型相转变为P3型相与Na + /空位有序耦合,这在O3型NaFeO 2中还没有观察到。用Co代替Fe可以稳定钠萃取形成的P3型相,并可以抑制O3型NaFeO 2中通常观察到的不可逆结构变化。 ,从而获得更好的循环保持率和更高的速率性能。尽管在中子衍射实验中未观察到过渡金属离子的有序化,但在蒙特卡罗模拟的支持下,通过Na + /空位有序化对Na 0.5 Fe 1的超晶格形成是由同步Na + /空位有序形成的/ 2 Co 1/2 O 2,这可能会在充电/放电曲线中涉及潜在的台阶。
更新日期:2016-06-23
中文翻译:

了解钠离子电池用NaFeO2–NaCoO2固溶体的结构演变和氧化还原机理
Na离子电池由于其丰富的Na资源而已成为大规模储能系统的有希望的候选者,并且由于其独特的行为(例如其对Fe 3+ / Fe 4+的氧化还原的电化学活性)已经引起了相当大的学术兴趣。夫妻。Na +离子的低路易斯酸度带来的高效率性能是Na-离子电池的另一个优势,并且已在NaFe 1/2 Co 1/2 O 2溶液中得到证明。这里是NaFeO 2 -NaCoO 2的固溶体合成了它们,并与它们各自的末端成员进行了比较,研究了其出色的电化学性能背后的机理。对Na 1– x Fe 1/2 Co 1/2 O 2的操作X射线衍射,非原位X射线吸收光谱和密度泛函理论(DFT)计算的综合分析显示,O3型相转变为P3型相与Na + /空位有序耦合,这在O3型NaFeO 2中还没有观察到。用Co代替Fe可以稳定钠萃取形成的P3型相,并可以抑制O3型NaFeO 2中通常观察到的不可逆结构变化。 ,从而获得更好的循环保持率和更高的速率性能。尽管在中子衍射实验中未观察到过渡金属离子的有序化,但在蒙特卡罗模拟的支持下,通过Na + /空位有序化对Na 0.5 Fe 1的超晶格形成是由同步Na + /空位有序形成的/ 2 Co 1/2 O 2,这可能会在充电/放电曲线中涉及潜在的台阶。




































 京公网安备 11010802027423号
京公网安备 11010802027423号