当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uranyl(VI) sorption in calcium silicate hydrate phases. A quantum chemical study of tobermorite models
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.apgeochem.2019.104463 Alena Kremleva , Sven Krüger , Notker Rösch
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.apgeochem.2019.104463 Alena Kremleva , Sven Krüger , Notker Rösch
|
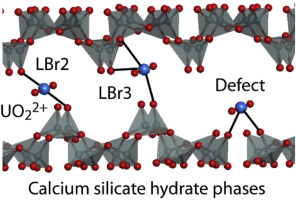
Abstract Using density functional theory, we present the first quantum chemical study on uranyl, UO22+, absorption into the interlayer region of calcium silicate hydrate (CSH) phases, the main constituents of fresh and degraded cement. We modeled CSH phases by tobermorite 14 A with various ratios of Ca/Si (C/S). Uranyl (VI) sorbed in the interlayer exhibits mainly coordination number 4. At higher C/S values, uranyl hydrolyzes and exhibits OH ligands in the first coordination shell. EXAFS data compare well with calculated structural features of sorption complexes. However, none of the sorption complexes determined displays all measured distances, suggesting that several sorption complexes co-exist. Our results represent the first comprehensive atomic scale model of actinyl sorption complexes in CSH and, together with pertinent EXAFS results, supports the hypothesis that interlayer absorption contributes to a considerable extent to the uptake of uranyl by CSH. Characteristic U–Si distances are assigned to the coordination mode of uranyl sorbed at SiO4 tetrahedra of CSH. Measured U–Ca distances appear due to U–O–Ca bridges, the number of which defines the U–Ca distance. We characterized the energy of UO22+ sorption by calculated exchange energies of Ca2+ or 2H+ by UO22+. For higher C/S this analysis reveals correlations between the preferred sites for uranyl sorption and the compensating charge, estimated empirically, of the functional groups forming the site. The sites with stronger compensation, mainly defect-derived and three-fold coordinative sites, were calculated to be the most favorable ones.
中文翻译:

硅酸钙水合物相中的铀酰 (VI) 吸附。雪硅钙石模型的量子化学研究
摘要 利用密度泛函理论,我们首次对铀酰、UO22+、硅酸钙水合物 (CSH) 相(新鲜和降解水泥的主要成分)的层间区域吸收进行了量子化学研究。我们通过具有不同 Ca/Si (C/S) 比率的雪硅钙石 14 A 模拟 CSH 相。吸附在夹层中的铀酰 (VI) 主要显示配位数 4。在较高的 C/S 值下,铀酰水解并在第一配位壳中显示出 OH 配体。EXAFS 数据与计算出的吸附复合物的结构特征相比较。然而,确定的吸附复合物没有一个显示所有测量的距离,这表明几种吸附复合物共存。我们的结果代表了 CSH 中肌动酰基吸附复合物的第一个综合原子尺度模型,以及相关的 EXAFS 结果,支持层间吸收在很大程度上有助于 CSH 吸收铀的假设。特征 U-Si 距离归于吸附在 CSH 的 SiO4 四面体上的铀酰的配位模式。测量的 U-Ca 距离是由于 U-O-Ca 桥而出现的,其数量定义了 U-Ca 距离。我们通过计算 UO22+ 与 Ca2+ 或 2H+ 的交换能来表征 UO22+ 吸附的能量。对于更高的 C/S,该分析揭示了铀酰吸附的首选位点与根据经验估计的形成该位点的官能团的补偿电荷之间的相关性。具有较强补偿的位点,主要是缺陷衍生位点和三重协调位点,被计算为最有利的位点。
更新日期:2020-02-01
中文翻译:

硅酸钙水合物相中的铀酰 (VI) 吸附。雪硅钙石模型的量子化学研究
摘要 利用密度泛函理论,我们首次对铀酰、UO22+、硅酸钙水合物 (CSH) 相(新鲜和降解水泥的主要成分)的层间区域吸收进行了量子化学研究。我们通过具有不同 Ca/Si (C/S) 比率的雪硅钙石 14 A 模拟 CSH 相。吸附在夹层中的铀酰 (VI) 主要显示配位数 4。在较高的 C/S 值下,铀酰水解并在第一配位壳中显示出 OH 配体。EXAFS 数据与计算出的吸附复合物的结构特征相比较。然而,确定的吸附复合物没有一个显示所有测量的距离,这表明几种吸附复合物共存。我们的结果代表了 CSH 中肌动酰基吸附复合物的第一个综合原子尺度模型,以及相关的 EXAFS 结果,支持层间吸收在很大程度上有助于 CSH 吸收铀的假设。特征 U-Si 距离归于吸附在 CSH 的 SiO4 四面体上的铀酰的配位模式。测量的 U-Ca 距离是由于 U-O-Ca 桥而出现的,其数量定义了 U-Ca 距离。我们通过计算 UO22+ 与 Ca2+ 或 2H+ 的交换能来表征 UO22+ 吸附的能量。对于更高的 C/S,该分析揭示了铀酰吸附的首选位点与根据经验估计的形成该位点的官能团的补偿电荷之间的相关性。具有较强补偿的位点,主要是缺陷衍生位点和三重协调位点,被计算为最有利的位点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号