当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chain Length Dependence of Hydrogen Bond Linkages between Cationic Constituents in Hydroxy-Functionalized Ionic Liquids: Tracking Bulk Behavior to the Molecular Level with Cold Cluster Ion Spectroscopy.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jpclett.9b03359
Helen J Zeng 1 , Fabian S Menges 1 , Thomas Niemann 2, 3 , Anne Strate 2, 3 , Ralf Ludwig 2, 3, 4 , Mark A Johnson 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jpclett.9b03359
Helen J Zeng 1 , Fabian S Menges 1 , Thomas Niemann 2, 3 , Anne Strate 2, 3 , Ralf Ludwig 2, 3, 4 , Mark A Johnson 1
Affiliation
|
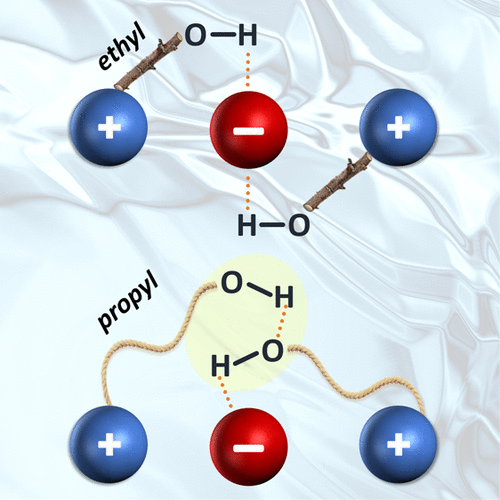
Hydroxy functionalization of cations in ionic liquids (ILs) can lead to formation of contacts between their OH groups [so-called (c-c) interactions]. One class of these linkages involves cooperatively enhanced hydrogen bonds to anionic partners that are sufficiently strong to overcome the repulsion between two positively charged centers. Herein, we clarify how the propensity for the formation of (c-c) contacts depends on the alkyl chain length between two cationic rings and their OH groups by analyzing the temperature-dependent IR spectra of bulk ILs as well as the vibrational predissociation spectra of ∼35 K complexes comprised of two cations and one anion. This study compares the behavior of two cationic derivatives with ethyl and propyl chains complexed with two different anions: bis(trifluoromethylsulfonyl)imide and tetrafluoroborate. Only the bulk ILs with the longer chain propyl derivative [HPMPip+ = 1-(3-hydroxypropyl)-1-methylpiperidinium] display (c-c) interactions. Molecular-level aspects of this docking arrangement are revealed by analyzing the OH stretching fundamentals displayed by the ternary complexes.
中文翻译:

羟基官能化离子液体中阳离子成分之间氢键键的链长依赖性:用冷簇离子光谱法在分子水平上追踪大分子行为。
阳离子在离子液体(ILs)中的羟基官能化可导致其OH基之间形成接触[所谓的(cc)相互作用]。这些连接中的一类涉及与阴离子配偶体的协同增强的氢键,该氢键足够牢固以克服两个带正电的中心之间的排斥。在此,我们通过分析体离子的温度依赖性红外光谱以及约35的振动预离解光谱,来阐明(cc)接触的倾向如何取决于两个阳离子环之间的烷基链长度及其OH基团K配合物由两个阳离子和一个阴离子组成。这项研究比较了带有两个不同阴离子的乙基和丙基链的两种阳离子衍生物的行为:双(三氟甲基磺酰基)酰亚胺和四氟硼酸酯。只有具有较长链丙基衍生物[HPMPip + = 1-(3-羟丙基)-1-甲基哌啶鎓]的本体IL表现出(cc)相互作用。通过分析三元配合物显示的OH拉伸基本原理可以揭示这种对接排列的分子水平方面。
更新日期:2020-01-15
中文翻译:

羟基官能化离子液体中阳离子成分之间氢键键的链长依赖性:用冷簇离子光谱法在分子水平上追踪大分子行为。
阳离子在离子液体(ILs)中的羟基官能化可导致其OH基之间形成接触[所谓的(cc)相互作用]。这些连接中的一类涉及与阴离子配偶体的协同增强的氢键,该氢键足够牢固以克服两个带正电的中心之间的排斥。在此,我们通过分析体离子的温度依赖性红外光谱以及约35的振动预离解光谱,来阐明(cc)接触的倾向如何取决于两个阳离子环之间的烷基链长度及其OH基团K配合物由两个阳离子和一个阴离子组成。这项研究比较了带有两个不同阴离子的乙基和丙基链的两种阳离子衍生物的行为:双(三氟甲基磺酰基)酰亚胺和四氟硼酸酯。只有具有较长链丙基衍生物[HPMPip + = 1-(3-羟丙基)-1-甲基哌啶鎓]的本体IL表现出(cc)相互作用。通过分析三元配合物显示的OH拉伸基本原理可以揭示这种对接排列的分子水平方面。

































 京公网安备 11010802027423号
京公网安备 11010802027423号