当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies of Pd(II)-Catalyzed Copolymerization of Ethylene and Vinylalkoxysilanes: Evidence for a β-Silyl Elimination Chain Transfer Mechanism
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-01 , DOI: 10.1021/jacs.6b10462
Zhou Chen 1 , Weijun Liu 2 , Olafs Daugulis 1 , Maurice Brookhart 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-01 , DOI: 10.1021/jacs.6b10462
Zhou Chen 1 , Weijun Liu 2 , Olafs Daugulis 1 , Maurice Brookhart 1, 2
Affiliation
|
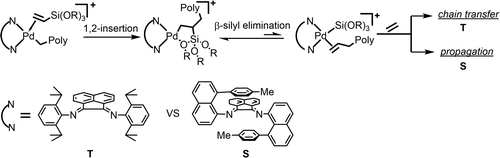
Copolymerizations of ethylene with vinyltrialkoxysilanes are reported using both a "traditional" cationic Pd(II) aryldiimine catalyst, t-1 (aryl = 2,6-diisopropylphenyl), and a "sandwich-type" aryldiimine catalyst, s-2 (aryl = 8-tolylnaphthyl). Incorporation levels of vinyltrialkoxysilanes between 0.25 and 2.0 mol % were achieved with remarkably little rate retardation relative to ethylene homopolymerizations. In the case of the traditional catalyst system, molecular weights decrease as the level of comonomer increases and only one trialkoxysilyl group is incorporated per chain. Molecular weight distributions of ca. 2 are observed. For the sandwich catalyst, higher molecular weights are observed with many more trialkoxysilyl groups incorporated per chain. Polymers with molecular weight distributions of ca. 1.2-1.4 are obtained. Detailed NMR mechanistic studies have revealed the formation of intermediate π-complexes of the type (diimine)Pd(alkyl)(vinyltrialkoxysilane)+. 1,2-Migratory insertions of these complexes occur with rates similar to ethylene insertion and result in formation of observable five-membered chelate intermediates. These chelates are rapidly opened with ethylene forming alkyl ethylene complexes, a requirement for chain growth. An unusual β-silyl elimination mechanism was shown to be responsible for chain transfer and formation of low molecular weight copolymers in the traditional catalyst system, t-1. This chain transfer process is retarded in the sandwich system. Relative binding affinities of ethylene and vinyltrialkoxysilanes to the cationic palladium center have been determined. The quantitative mechanistic studies reported fully explain the features of the bulk polymerization results.
中文翻译:

Pd(II)-催化乙烯和乙烯基烷氧基硅烷共聚的机理研究:β-甲硅烷基消除链转移机制的证据
据报道,使用“传统”阳离子 Pd(II) 芳基二亚胺催化剂 t-1(芳基 = 2,6-二异丙基苯基)和“夹心型”芳基二亚胺催化剂 s-2(芳基 = 8-甲苯基萘基)。乙烯基三烷氧基硅烷的掺入水平在 0.25 至 2.0 mol% 之间,相对于乙烯均聚反应的速率延迟非常小。在传统催化剂体系的情况下,分子量随着共聚单体含量的增加而降低,并且每条链仅引入一个三烷氧基甲硅烷基。分子量分布约。2 被观察到。对于夹心催化剂,观察到更高的分子量,每条链引入了更多的三烷氧基甲硅烷基。分子量分布为约的聚合物。得到1.2-1.4。详细的 NMR 机理研究揭示了(二亚胺)Pd(烷基)(乙烯基三烷氧基硅烷)+ 类型的中间 π 配合物的形成。这些复合物的 1,2-迁移插入以类似于乙烯插入的速率发生,并导致形成可观察到的五元螯合中间体。这些螯合物与乙烯一起迅速打开,形成烷基乙烯配合物,这是链增长的必要条件。在传统的催化剂体系 t-1 中,一种不寻常的 β-甲硅烷基消除机制被证明是导致链转移和形成低分子量共聚物的原因。这种链转移过程在夹心系统中被延迟。乙烯和乙烯基三烷氧基硅烷对阳离子钯中心的相对结合亲和力已经确定。
更新日期:2016-12-01
中文翻译:

Pd(II)-催化乙烯和乙烯基烷氧基硅烷共聚的机理研究:β-甲硅烷基消除链转移机制的证据
据报道,使用“传统”阳离子 Pd(II) 芳基二亚胺催化剂 t-1(芳基 = 2,6-二异丙基苯基)和“夹心型”芳基二亚胺催化剂 s-2(芳基 = 8-甲苯基萘基)。乙烯基三烷氧基硅烷的掺入水平在 0.25 至 2.0 mol% 之间,相对于乙烯均聚反应的速率延迟非常小。在传统催化剂体系的情况下,分子量随着共聚单体含量的增加而降低,并且每条链仅引入一个三烷氧基甲硅烷基。分子量分布约。2 被观察到。对于夹心催化剂,观察到更高的分子量,每条链引入了更多的三烷氧基甲硅烷基。分子量分布为约的聚合物。得到1.2-1.4。详细的 NMR 机理研究揭示了(二亚胺)Pd(烷基)(乙烯基三烷氧基硅烷)+ 类型的中间 π 配合物的形成。这些复合物的 1,2-迁移插入以类似于乙烯插入的速率发生,并导致形成可观察到的五元螯合中间体。这些螯合物与乙烯一起迅速打开,形成烷基乙烯配合物,这是链增长的必要条件。在传统的催化剂体系 t-1 中,一种不寻常的 β-甲硅烷基消除机制被证明是导致链转移和形成低分子量共聚物的原因。这种链转移过程在夹心系统中被延迟。乙烯和乙烯基三烷氧基硅烷对阳离子钯中心的相对结合亲和力已经确定。





































 京公网安备 11010802027423号
京公网安备 11010802027423号