当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of 3-(4-Aryl-1H-1,2,3-triazol-1-yl)-Biphenyl Derivatives as P2Y14 Receptor Antagonists.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-06-22 , DOI: 10.1021/acs.jmedchem.6b00044 Anna Junker 1 , Ramachandran Balasubramanian 1 , Antonella Ciancetta 1 , Elisa Uliassi 1 , Evgeny Kiselev 1 , Chiara Martiriggiano 1 , Kevin Trujillo 1 , Giorgi Mtchedlidze 1 , Leah Birdwell 1 , Kyle A Brown 2 , T Kendall Harden 2 , Kenneth A Jacobson 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-06-22 , DOI: 10.1021/acs.jmedchem.6b00044 Anna Junker 1 , Ramachandran Balasubramanian 1 , Antonella Ciancetta 1 , Elisa Uliassi 1 , Evgeny Kiselev 1 , Chiara Martiriggiano 1 , Kevin Trujillo 1 , Giorgi Mtchedlidze 1 , Leah Birdwell 1 , Kyle A Brown 2 , T Kendall Harden 2 , Kenneth A Jacobson 1
Affiliation
|
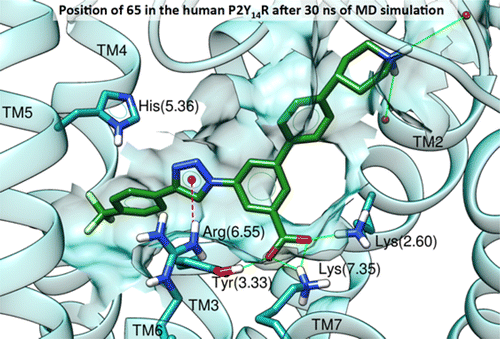
UDP and UDP-glucose activate the P2Y14 receptor (P2Y14R) to modulate processes related to inflammation, diabetes, and asthma. A computational pipeline suggested alternatives to naphthalene of a previously reported P2Y14R antagonist (3, PPTN) using docking and molecular dynamics simulations on a hP2Y14R homology model based on P2Y12R structures. By reevaluating the binding of 3 to P2Y14R computationally, two alternatives, i.e., alkynyl and triazolyl derivatives, were identified. Improved synthesis of fluorescent antagonist 4 enabled affinity quantification (IC50s, nM) using flow cytometry of P2Y14R-expressing CHO cells. p-F3C-phenyl-triazole 65 (32) was more potent than a corresponding alkyne 11. Thus, additional triazolyl derivatives were prepared, as guided by docking simulations, with nonpolar aryl substituents favored. Although triazoles were less potent than 3 (6), simpler synthesis facilitated further structural optimization. Additionally, relative P2Y14R affinities agreed with predicted binding of alkynyl and triazole analogues. These triazoles, designed through a structure-based approach, can be assessed in disease models.
中文翻译:

基于结构的3-(4-芳基-1H-1,2,3-三唑-1-基)-联苯衍生物作为P2Y14受体拮抗剂的设计。
UDP和UDP葡萄糖激活P2Y14受体(P2Y14R),以调节与炎症,糖尿病和哮喘有关的过程。计算流水线建议在基于P2Y12R结构的hP2Y14R同源性模型上使用对接和分子动力学模拟,以替代先前报道的P2Y14R拮抗剂(3,PPTN)中的萘。通过重新计算3与P2Y14R的结合,确定了两个替代方案,即炔基和三唑基衍生物。使用表达P2Y14R的CHO细胞的流式细胞术,可以改善荧光拮抗剂4的合成亲和力(IC50s,nM)。对-F 3 C-苯基-三唑65(32)比相应的炔烃11更有效。因此,在对接模拟的指导下,制备了额外的三唑基衍生物,其中偏极性芳基取代基受到青睐。尽管三唑的效力不及3(6),但更简单的合成促进了进一步的结构优化。另外,相对的P2Y14R亲和力与炔基和三唑类似物的预测结合一致。这些三唑是通过基于结构的方法设计的,可以在疾病模型中进行评估。
更新日期:2016-06-22
中文翻译:

基于结构的3-(4-芳基-1H-1,2,3-三唑-1-基)-联苯衍生物作为P2Y14受体拮抗剂的设计。
UDP和UDP葡萄糖激活P2Y14受体(P2Y14R),以调节与炎症,糖尿病和哮喘有关的过程。计算流水线建议在基于P2Y12R结构的hP2Y14R同源性模型上使用对接和分子动力学模拟,以替代先前报道的P2Y14R拮抗剂(3,PPTN)中的萘。通过重新计算3与P2Y14R的结合,确定了两个替代方案,即炔基和三唑基衍生物。使用表达P2Y14R的CHO细胞的流式细胞术,可以改善荧光拮抗剂4的合成亲和力(IC50s,nM)。对-F 3 C-苯基-三唑65(32)比相应的炔烃11更有效。因此,在对接模拟的指导下,制备了额外的三唑基衍生物,其中偏极性芳基取代基受到青睐。尽管三唑的效力不及3(6),但更简单的合成促进了进一步的结构优化。另外,相对的P2Y14R亲和力与炔基和三唑类似物的预测结合一致。这些三唑是通过基于结构的方法设计的,可以在疾病模型中进行评估。






























 京公网安备 11010802027423号
京公网安备 11010802027423号