当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Catalytic Oxidation of Unprotected Carbohydrates
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-20 00:00:00 , DOI: 10.1021/acscatal.6b01501 Kevin Chung 1 , Robert M. Waymouth 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-20 00:00:00 , DOI: 10.1021/acscatal.6b01501 Kevin Chung 1 , Robert M. Waymouth 1
Affiliation
|
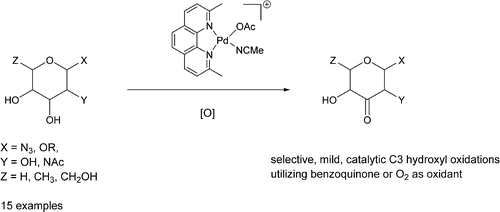
The development of new strategies for the direct catalytic functionalization of unprotected carbohydrates would be an enabling advance for glycoscience. Herein we report that the catalytic oxidation of unprotected carbohydrates can be carried out selectively with [(neocuproine)Pd(OAc)]2(OTf)2 (1) to generate the 3-ketoses. Catalytic aerobic oxidation can be carried out with Pd loadings as low as 1% in the presence of phenolic additives. Catalytic oxidation of a variety of unprotected pyranosides in acetonitrile or acetonitrile/water with Pd catalyst 1 with either oxygen or benzoquinone selectively generates the 3-ketoses. Minor amounts of the 4-ketoses are formed competitively, particularly in the case of pyranosides bearing axial substituents at C4 of the pyranoside. Catalytic oxidations can also be carried out in trifluorethanol, but for pyranosides bearing axial substituents at C2 or C4, selective oxidation to the 3-ketose is accompanied by epimerization to afford the equatorial 3-ketoses. Catalytic oxidation of unprotected hexafuranosides or sialic acid derivatives occurs selectively at the exocyclic diol or triol in trifluoroethanol to generate exocyclic hydroxyketones.
中文翻译:

未保护的碳水化合物的选择性催化氧化
对未保护的碳水化合物进行直接催化功能化的新策略的开发将是糖科学的一个有利的进展。在本文中,我们报道了未保护的碳水化合物的催化氧化可以选择性地与[[neocuproine)Pd(OAc)] 2(OTf)2(1)进行,以生成3-酮糖。在酚类添加剂存在下,Pd含量低至1%即可进行催化好氧氧化。使用Pd催化剂1催化乙腈或乙腈/水中的各种未保护吡喃糖苷的催化氧化1用氧气或苯醌选择性地生成3-酮糖。少量的4-酮糖竞争性地形成,特别是在吡喃糖苷在吡喃糖苷的C4处带有轴向取代基的情况下。催化氧化也可以在三氟乙醇中进行,但是对于在C2或C4处带有轴向取代基的吡喃糖苷,选择性氧化成3-酮糖伴随着差向异构化作用,得到了赤道的3-酮糖。未保护的六呋喃糖苷或唾液酸衍生物的催化氧化选择性地在三氟乙醇中的环外二醇或三醇处发生,以生成环外羟基酮。
更新日期:2016-06-20
中文翻译:

未保护的碳水化合物的选择性催化氧化
对未保护的碳水化合物进行直接催化功能化的新策略的开发将是糖科学的一个有利的进展。在本文中,我们报道了未保护的碳水化合物的催化氧化可以选择性地与[[neocuproine)Pd(OAc)] 2(OTf)2(1)进行,以生成3-酮糖。在酚类添加剂存在下,Pd含量低至1%即可进行催化好氧氧化。使用Pd催化剂1催化乙腈或乙腈/水中的各种未保护吡喃糖苷的催化氧化1用氧气或苯醌选择性地生成3-酮糖。少量的4-酮糖竞争性地形成,特别是在吡喃糖苷在吡喃糖苷的C4处带有轴向取代基的情况下。催化氧化也可以在三氟乙醇中进行,但是对于在C2或C4处带有轴向取代基的吡喃糖苷,选择性氧化成3-酮糖伴随着差向异构化作用,得到了赤道的3-酮糖。未保护的六呋喃糖苷或唾液酸衍生物的催化氧化选择性地在三氟乙醇中的环外二醇或三醇处发生,以生成环外羟基酮。















































 京公网安备 11010802027423号
京公网安备 11010802027423号