当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predissociation measurements of bond dissociation energies: VC, VN, and VS
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-06-16 12:19:36 , DOI: 10.1063/1.4953782 Eric L. Johnson 1 , Quincy C. Davis 1 , Michael D. Morse 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-06-16 12:19:36 , DOI: 10.1063/1.4953782 Eric L. Johnson 1 , Quincy C. Davis 1 , Michael D. Morse 1
Affiliation
|
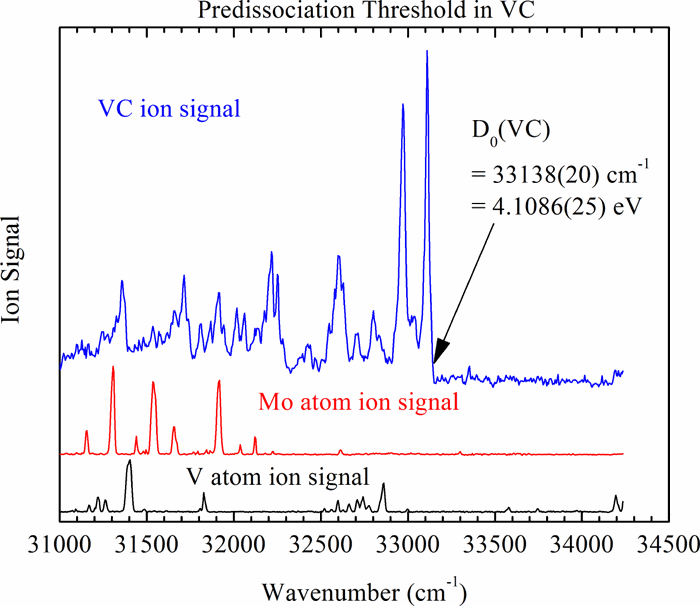
The abrupt onset of predissociation in the congested electronic spectra of jet-cooled VC, VN, and VS has been observed using resonant two-photon ionization spectroscopy. It is argued that because of the high density of electronic states in these molecules, the predissociation threshold occurs at the thermochemical threshold for the production of separated atoms in their ground electronic states. As a result, the measured threshold represents the bond dissociation energy. Using this method, bond dissociation energies of D0(V C) = 4.1086(25) eV, D0(V N) = 4.9968(20) eV, and D0(V S) = 4.5353(25) eV are obtained. From these values, enthalpies of formation are derived as Δf,0KH°(V C(g)) = 827.0 ± 8 kJ mol−1, Δf,0KH°(V N(g)) = 500.9 ± 8 kJ mol−1, and Δf,0KH°(V S(g)) = 349.3 ± 8 kJ mol−1. Using a thermochemical cycle and the well-known ionization energies of V, VC, and VN, our results also provide D0(V+–C) = 3.7242(25) eV and D0(V+–N) = 4.6871(20) eV. These values are compared to previous measurements and to computational results. The precision of these bond dissociation energies makes them good candidates for testing computational chemistry methods, particularly those that employ density functional theory.
中文翻译:

键解离能的预解离测量:VC,VN和VS
喷射冷却的VC,VN和VS的电子拥挤谱中预离解的突然发生已使用共振双光子电离光谱法进行了观察。据认为,由于这些分子中电子态的高密度,预离解阈值发生在热化学阈值上,以产生其基础电子态的分离原子。结果,测得的阈值代表键离解能。使用此方法,可以获得键解离能D 0(VC)= 4.1086(25)eV,D 0(VN)= 4.9968(20)eV和D 0(VS)= 4.5353(25)eV。根据这些值,形成焓为Δf,0K H°(VC(g))= 827.0±8 kJ mol -1,Δf,0K H°(VN(g))= 500.9±8 kJ mol -1,Δf,0K H°(VS(g))= 349.3±8 kJ mol -1。使用热化学循环和众所周知的V,VC和VN的电离能,我们的结果还提供了D 0(V + –C)= 3.7242(25)eV和D 0(V + –N)= 4.6871(20) )eV。将这些值与先前的测量结果和计算结果进行比较。这些键解离能的精确度使其成为测试计算化学方法的良好候选者,特别是那些采用密度泛函理论的方法。
更新日期:2016-06-17
中文翻译:

键解离能的预解离测量:VC,VN和VS
喷射冷却的VC,VN和VS的电子拥挤谱中预离解的突然发生已使用共振双光子电离光谱法进行了观察。据认为,由于这些分子中电子态的高密度,预离解阈值发生在热化学阈值上,以产生其基础电子态的分离原子。结果,测得的阈值代表键离解能。使用此方法,可以获得键解离能D 0(VC)= 4.1086(25)eV,D 0(VN)= 4.9968(20)eV和D 0(VS)= 4.5353(25)eV。根据这些值,形成焓为Δf,0K H°(VC(g))= 827.0±8 kJ mol -1,Δf,0K H°(VN(g))= 500.9±8 kJ mol -1,Δf,0K H°(VS(g))= 349.3±8 kJ mol -1。使用热化学循环和众所周知的V,VC和VN的电离能,我们的结果还提供了D 0(V + –C)= 3.7242(25)eV和D 0(V + –N)= 4.6871(20) )eV。将这些值与先前的测量结果和计算结果进行比较。这些键解离能的精确度使其成为测试计算化学方法的良好候选者,特别是那些采用密度泛函理论的方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号