当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Thailanstatin A
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-06-13 , DOI: 10.1021/jacs.6b04781 K. C. Nicolaou 1 , Derek Rhoades , Manjunath Lamani , Manas R. Pattanayak , S. Mothish Kumar
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-06-13 , DOI: 10.1021/jacs.6b04781 K. C. Nicolaou 1 , Derek Rhoades , Manjunath Lamani , Manas R. Pattanayak , S. Mothish Kumar
Affiliation
|
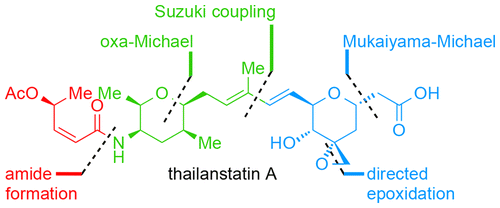
The total synthesis of the spliceosome inhibitor thailanstatin A has been achieved in a longest linear sequence of nine steps from readily available starting materials. A key feature of the developed synthetic strategy is the implementation of a unique, biomimetic asymmetric intramolecular oxa-Michael reaction/hydrogenation sequence that allows diastereodivergent access to highly functionalized tetrahydropyrans, which can be used for the synthesis of designed analogues of this bioactive molecule.
中文翻译:

Thailanstatin A的全合成
剪接体抑制剂泰兰他汀 A 的全合成已从现成的起始材料中以最长的 9 步线性序列实现。所开发的合成策略的一个关键特征是实施独特的仿生不对称分子内氧杂-迈克尔反应/氢化序列,允许非对映发散获得高度官能化的四氢吡喃,可用于合成该生物活性分子的设计类似物。
更新日期:2016-06-13
中文翻译:

Thailanstatin A的全合成
剪接体抑制剂泰兰他汀 A 的全合成已从现成的起始材料中以最长的 9 步线性序列实现。所开发的合成策略的一个关键特征是实施独特的仿生不对称分子内氧杂-迈克尔反应/氢化序列,允许非对映发散获得高度官能化的四氢吡喃,可用于合成该生物活性分子的设计类似物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号