当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A One-Step Synthesis of C6 Sugar Alcohols from Levoglucosan and Disaccharides Using a Ru/CMK-3 Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-13 00:00:00 , DOI: 10.1021/acscatal.6b00296 Wang Yin 1 , Zhenchen Tang 1 , Robertus Hendrikus Venderbosch 2 , Zheng Zhang 1 , Catia Cannilla 3 , Giuseppe Bonura 3 , Francesco Frusteri 3 , Hero Jan Heeres 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-13 00:00:00 , DOI: 10.1021/acscatal.6b00296 Wang Yin 1 , Zhenchen Tang 1 , Robertus Hendrikus Venderbosch 2 , Zheng Zhang 1 , Catia Cannilla 3 , Giuseppe Bonura 3 , Francesco Frusteri 3 , Hero Jan Heeres 1
Affiliation
|
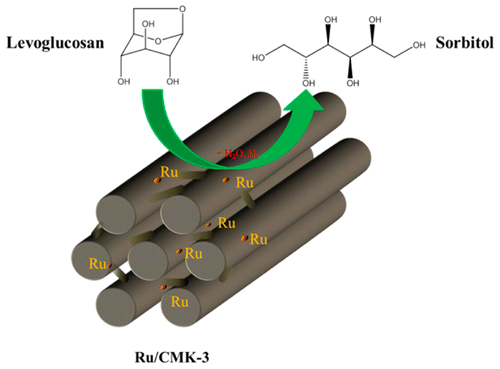
Sorbitol is an important commercially available chemical with a broad application range and is typically made by the catalytic hydrogenation of glucose. Here we report a high-yield synthesis of sorbitol from levoglucosan (1,6-anhydro-β-d-glucopyranose) and cellobiose, two sugars present in pyrolysis liquids, using a mesoporous carbon-supported Ru catalyst (Ru/CMK-3). The hydrogenation reactions were performed in a batch autoclave setup under a hydrogen pressure of 50 bar and temperatures ranging from 120 to 180 °C in water. The hydrogenation of levoglucosan gave essentially quantitative yields of sugar alcohols, composed of 96.2 wt % of sorbitol and 3.8 wt % of mannitol (180 °C, 5 h). Ru/CMK-3 shows superior catalytic performance compared to a commercial Ru/C catalyst. A reaction pathway involving glucose as an intermediate and subsequent (hydrogenolysis) reactions of the desired sorbitol is proposed. Reactions with glucose and sorbitol were performed to define the reaction pathways and to highlight the differences between Ru/C and Ru/CMK-3. Disaccharides including cellobiose and sucrose were also tested, yielding up to 95 wt % of C6 sugar alcohols at 180 °C in 5 h for both substrates. Detailed catalyst characterization studies (N2 physisorption, TEM, XRD, NH3-TPD, H2-TPD) revealed that Ru/CMK-3 contains considerable amounts of strong acid sites (NH3-TPD). Catalyst stability was tested by catalyst recycling experiments using levoglucosan in batch. After three successive runs, the rate of the hydrolysis reaction of LG to glucose was about constant, though the subsequent hydrogenation reaction to sorbitol/mannitol was slightly retarded as evidenced from a slight increase in the remaining amounts of glucose at the end of reaction.
中文翻译:

Ru / CMK-3催化剂从左旋葡聚糖和二糖一步合成C6糖醇
山梨糖醇是一种重要的可商购获得的化学品,具有广泛的应用范围,通常是通过葡萄糖的催化加氢制得的。在这里我们报道了从左旋葡聚糖(1,6-anhydro-β- d-吡喃葡萄糖和纤维二糖,这两种糖存在于热解液体中,使用介孔碳负载的Ru催化剂(Ru / CMK-3)。氢化反应在间歇高压釜中在50 bar的氢气压力和120至180°C的水中温度下进行。左旋葡聚糖的氢化基本上得到定量的糖醇,其由96.2重量%的山梨糖醇和3.8重量%的甘露糖醇组成(180℃,5小时)。与商业化的Ru / C催化剂相比,Ru / CMK-3表现出优异的催化性能。提出了一种反应途径,其涉及葡萄糖作为所需山梨糖醇的中间和随后的(氢解)反应。进行与葡萄糖和山梨糖醇的反应以定义反应路径并突出Ru / C和Ru / CMK-3之间的差异。还测试了包括纤维二糖和蔗糖在内的二糖,两种底物在180°C下于5小时内产生高达95 wt%的C6糖醇。详细的催化剂表征研究(N2物理吸附,TEM,XRD,NH 3 -TPD,H 2 -TPD)表明Ru / CMK-3含有大量的强酸位点(NH 3 -TPD)。催化剂的稳定性通过分批使用左旋葡聚糖的催化剂再循环实验进行测试。经过三轮连续运行后,LG水解为葡萄糖的速率基本保持不变,尽管随后反应生成山梨糖醇/甘露糖醇的氢化反应略有延迟,反应结束时葡萄糖的残留量略有增加,这证明了这一点。
更新日期:2016-06-13
中文翻译:

Ru / CMK-3催化剂从左旋葡聚糖和二糖一步合成C6糖醇
山梨糖醇是一种重要的可商购获得的化学品,具有广泛的应用范围,通常是通过葡萄糖的催化加氢制得的。在这里我们报道了从左旋葡聚糖(1,6-anhydro-β- d-吡喃葡萄糖和纤维二糖,这两种糖存在于热解液体中,使用介孔碳负载的Ru催化剂(Ru / CMK-3)。氢化反应在间歇高压釜中在50 bar的氢气压力和120至180°C的水中温度下进行。左旋葡聚糖的氢化基本上得到定量的糖醇,其由96.2重量%的山梨糖醇和3.8重量%的甘露糖醇组成(180℃,5小时)。与商业化的Ru / C催化剂相比,Ru / CMK-3表现出优异的催化性能。提出了一种反应途径,其涉及葡萄糖作为所需山梨糖醇的中间和随后的(氢解)反应。进行与葡萄糖和山梨糖醇的反应以定义反应路径并突出Ru / C和Ru / CMK-3之间的差异。还测试了包括纤维二糖和蔗糖在内的二糖,两种底物在180°C下于5小时内产生高达95 wt%的C6糖醇。详细的催化剂表征研究(N2物理吸附,TEM,XRD,NH 3 -TPD,H 2 -TPD)表明Ru / CMK-3含有大量的强酸位点(NH 3 -TPD)。催化剂的稳定性通过分批使用左旋葡聚糖的催化剂再循环实验进行测试。经过三轮连续运行后,LG水解为葡萄糖的速率基本保持不变,尽管随后反应生成山梨糖醇/甘露糖醇的氢化反应略有延迟,反应结束时葡萄糖的残留量略有增加,这证明了这一点。































 京公网安备 11010802027423号
京公网安备 11010802027423号