当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong Impact of Platinum Surface Structure on Primary and Secondary Alcohol Oxidation during Electro-Oxidation of Glycerol
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-13 00:00:00 , DOI: 10.1021/acscatal.6b00709
Amanda C. Garcia 1, 2 , Manuel J. Kolb 1 , Chris van Nierop y Sanchez 1 , Jan Vos 1 , Yuvraj Y. Birdja 1 , Youngkook Kwon 1 , Germano Tremiliosi-Filho 2 , Marc T. M. Koper 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-13 00:00:00 , DOI: 10.1021/acscatal.6b00709
Amanda C. Garcia 1, 2 , Manuel J. Kolb 1 , Chris van Nierop y Sanchez 1 , Jan Vos 1 , Yuvraj Y. Birdja 1 , Youngkook Kwon 1 , Germano Tremiliosi-Filho 2 , Marc T. M. Koper 1
Affiliation
|
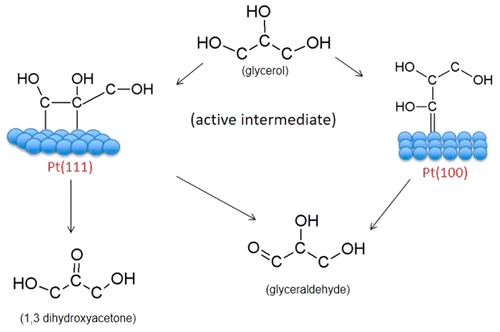
Herein we describe a combined experimental and computational study of electrochemical glycerol oxidation in acidic media on Pt(111) and Pt(100) electrodes. Our results show that glycerol oxidation is a very structure-sensitive reaction in terms of activity and, more surprisingly, in terms of selectivity. Using a combination of online HPLC and online electrochemical mass spectrometry, we show that on the Pt(111) electrode, glyceraldehyde, glyceric acid, and dihydroxyacetone are products of glycerol oxidation, while on the Pt(100) electrode, only glyceraldehyde was detected as the main product of the reaction. Density functional theory calculations show that this difference in selectivity is explained by different binding modes of dehydrogenated glycerol to the two surfaces. On Pt(111), the dehydrogenated glycerol intermediate binds to the surface through two single Pt–C bonds, yielding an enediol-like intermediate, which serves as a precursor to both glyceraldehyde and dihydroxyacetone. On Pt(100), the dehydrogenated glycerol intermediate binds to the surface through one double Pt═C bond, yielding glyceraldehyde as the only product. Stripping and in situ FTIR measurements show that CO is not the only strongly bound adsorbed intermediate of the oxidation of glycerol, glyceraldehyde, and dihydroxyacetone. Although the nature of this adsorbate is still unclear, this intermediate is highly resistant to oxidation and can only be removed from the Pt surface after multiple scans.
中文翻译:

甘油电氧化过程中铂表面结构对一次和二次醇氧化的强烈影响
在本文中,我们描述了在酸性介质中Pt(111)和Pt(100)电极上电化学甘油氧化的组合实验和计算研究。我们的结果表明,就活性而言,甘油氧化是对结构非常敏感的反应,更令人惊奇的是,就选择性而言。使用在线HPLC和在线电化学质谱联用,我们显示在Pt(111)电极上,甘油醛,甘油酸和二羟基丙酮是甘油氧化的产物,而在Pt(100)电极上,仅检测到甘油醛为反应的主要产物。密度泛函理论计算表明,选择性的差异是由脱氢甘油在两个表面上的不同结合方式所解释的。在铂(111)上,脱氢的甘油中间体通过两个单个的Pt-C键与表面结合,生成类似eneenediol的中间体,可作为甘油醛和二羟基丙酮的前体。在Pt(100)上,脱氢甘油中间体通过一个双Pt═C键与表面结合,从而产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。脱氢甘油中间体通过一个双Pt═C键与表面结合,产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。脱氢甘油中间体通过一个双Pt═C键与表面结合,产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。
更新日期:2016-06-13
中文翻译:

甘油电氧化过程中铂表面结构对一次和二次醇氧化的强烈影响
在本文中,我们描述了在酸性介质中Pt(111)和Pt(100)电极上电化学甘油氧化的组合实验和计算研究。我们的结果表明,就活性而言,甘油氧化是对结构非常敏感的反应,更令人惊奇的是,就选择性而言。使用在线HPLC和在线电化学质谱联用,我们显示在Pt(111)电极上,甘油醛,甘油酸和二羟基丙酮是甘油氧化的产物,而在Pt(100)电极上,仅检测到甘油醛为反应的主要产物。密度泛函理论计算表明,选择性的差异是由脱氢甘油在两个表面上的不同结合方式所解释的。在铂(111)上,脱氢的甘油中间体通过两个单个的Pt-C键与表面结合,生成类似eneenediol的中间体,可作为甘油醛和二羟基丙酮的前体。在Pt(100)上,脱氢甘油中间体通过一个双Pt═C键与表面结合,从而产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。脱氢甘油中间体通过一个双Pt═C键与表面结合,产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。脱氢甘油中间体通过一个双Pt═C键与表面结合,产生甘油醛作为唯一产物。汽提和原位FTIR测量表明,CO并不是甘油,甘油醛和二羟基丙酮氧化的唯一牢固结合的吸附中间体。尽管这种被吸附物的性质尚不清楚,但该中间体具有很高的抗氧化性,只能在多次扫描后才能从Pt表面去除。




































 京公网安备 11010802027423号
京公网安备 11010802027423号