当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of NOD2 and its implications in human disease.
Nature Communications ( IF 14.7 ) Pub Date : 2016-06-10 , DOI: 10.1038/ncomms11813
Sakiko Maekawa , Umeharu Ohto , Takuma Shibata , Kensuke Miyake , Toshiyuki Shimizu
Nature Communications ( IF 14.7 ) Pub Date : 2016-06-10 , DOI: 10.1038/ncomms11813
Sakiko Maekawa , Umeharu Ohto , Takuma Shibata , Kensuke Miyake , Toshiyuki Shimizu
|
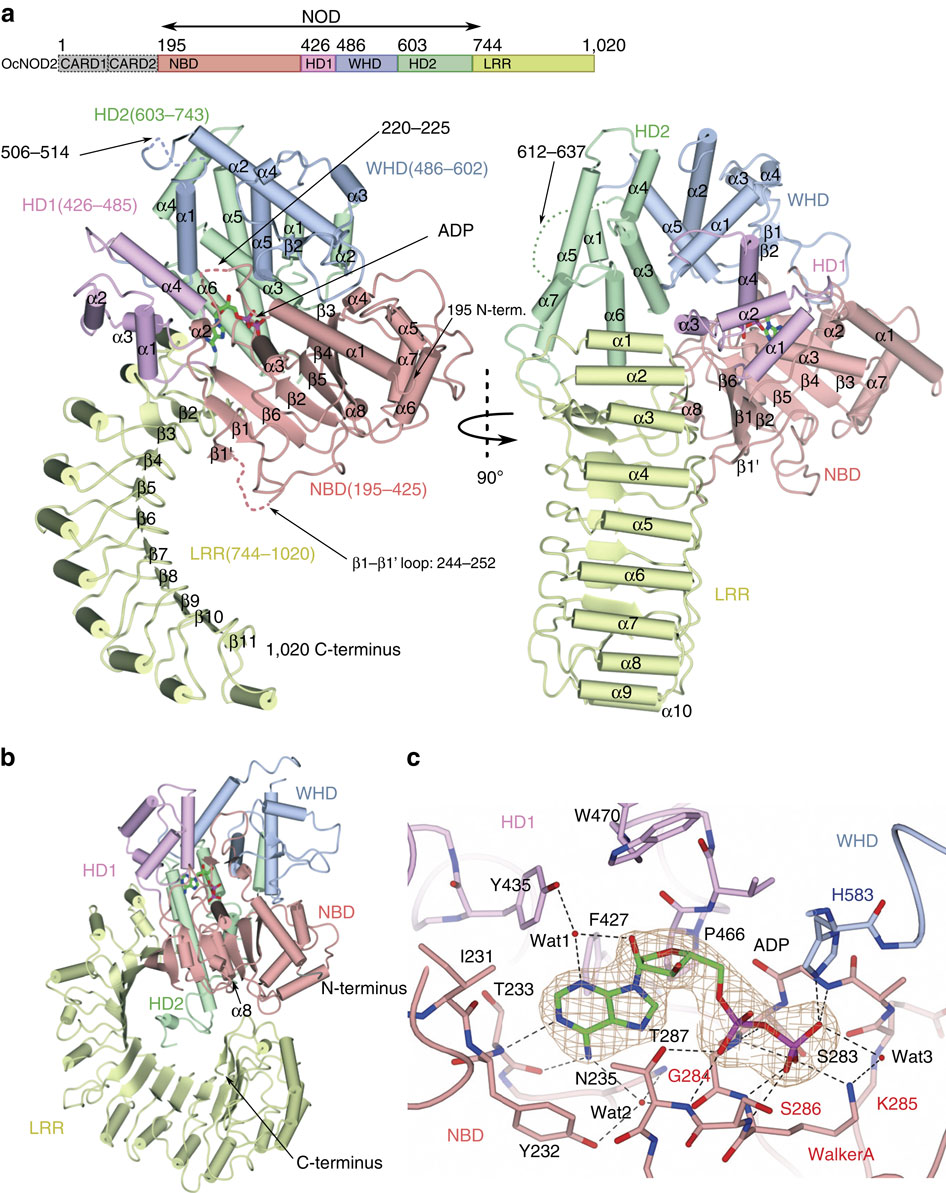
Nucleotide-binding oligomerization domain-containing protein 2 (NOD2), a member of the NOD-like receptors family, are crucial for innate immune responses. Mutations of NOD2 have been associated with chronic inflammatory disorders such as Crohn's disease (CD), Blau syndrome (BS) and early-onset sarcoidosis (EOS), but little is known about its signalling mechanism and the role it plays in these diseases. Here, we report the crystal structure of rabbit NOD2 in an ADP-bound state. The structure reveals an inactive closed conformation in which the subdomains in the NOD domain are closely packed by ADP-mediated and inter-domain interactions. Mapping of the BS- or EOS-associated gain-of-function mutations reveals that most of these mutations are located in the NOD subdomain interfaces, and are likely to disrupt the inner domain interactions, facilitating a conformational change to the active form. Conversely, mutations associated with CD are distributed throughout the protein, some of which may affect oligomer formation and ligand binding.
中文翻译:

NOD2的晶体结构及其对人类疾病的影响。
含有核苷酸结合的寡聚域的蛋白质2(NOD2)是NOD样受体家族的成员,对于先天免疫反应至关重要。NOD2突变与慢性炎症性疾病(如克罗恩病(CD),布劳综合征(BS)和早发结节病(EOS))有关,但对其信号传导机制及其在这些疾病中的作用知之甚少。在这里,我们报告在ADP绑定状态的兔子NOD2的晶体结构。该结构揭示了一种非活性的封闭构象,其中NOD域中的子域通过ADP介导的域间相互作用而紧密堆积。与BS或EOS相关的功能获得性突变的图谱显示,这些突变大多数位于NOD子域界面中,并且很可能破坏内部域的相互作用,促进构型改变有效形式。相反,与CD相关的突变分布在整个蛋白质中,其中一些可能影响寡聚物形成和配体结合。
更新日期:2016-06-13
中文翻译:

NOD2的晶体结构及其对人类疾病的影响。
含有核苷酸结合的寡聚域的蛋白质2(NOD2)是NOD样受体家族的成员,对于先天免疫反应至关重要。NOD2突变与慢性炎症性疾病(如克罗恩病(CD),布劳综合征(BS)和早发结节病(EOS))有关,但对其信号传导机制及其在这些疾病中的作用知之甚少。在这里,我们报告在ADP绑定状态的兔子NOD2的晶体结构。该结构揭示了一种非活性的封闭构象,其中NOD域中的子域通过ADP介导的域间相互作用而紧密堆积。与BS或EOS相关的功能获得性突变的图谱显示,这些突变大多数位于NOD子域界面中,并且很可能破坏内部域的相互作用,促进构型改变有效形式。相反,与CD相关的突变分布在整个蛋白质中,其中一些可能影响寡聚物形成和配体结合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号