当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
With DBU-activated N-bromophthalimide as potential N-sources to achieve P–N cross-coupling of P(O)–H compounds
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-06-06 16:06:51 Yueju Li, Fushun Liang
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-06-06 16:06:51 Yueju Li, Fushun Liang
|
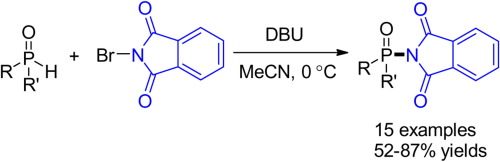
We have demonstrated that N-haloimides can be utilized as an aminating reagent, in addition to the traditional brominating reagent. Herein, the P–N cross-coupling of P(O)–H compounds and N-bromophthalimide has been developed with the DBU activation strategy. The nitrogen source may arise from internal N-bromophthalimide itself (a self-immolating reagent) or external phthalimide derivatives (with NBS-DBU activation system). The reaction features broad phosphorous compounds scope, including H-phosphinates, H-phosphonates, and H-phosphine oxides, and short reaction time (less than 10min). This method provides a simple, convenient, efficient, and green route toward phosphoramidates which are of biological importance under mild conditions.
中文翻译:

用DBU活化的N-溴邻苯二甲酰亚胺作为潜在的N源,实现P(O)-H化合物的PN交叉偶联
我们已经证明,除了传统的溴化试剂之外,N-卤代酰亚胺还可以用作胺化试剂。在本文中,已通过DBU活化策略开发了P(O)-H化合物与N-溴邻苯二甲酰亚胺的P–N交叉偶联。氮源可能来自内部N-溴邻苯二甲酰亚胺本身(自消灭试剂)或外部邻苯二甲酰亚胺衍生物(具有NBS-DBU活化系统)。该反应具有宽泛的磷化合物范围,包括H-次膦酸酯,H-膦酸酯和H-膦氧化物,并且反应时间短(少于10分钟)。该方法为在温和条件下具有生物学重要性的氨基磷酸酯提供了一种简单,方便,有效和绿色的途径。
更新日期:2016-06-07
中文翻译:

用DBU活化的N-溴邻苯二甲酰亚胺作为潜在的N源,实现P(O)-H化合物的PN交叉偶联
我们已经证明,除了传统的溴化试剂之外,N-卤代酰亚胺还可以用作胺化试剂。在本文中,已通过DBU活化策略开发了P(O)-H化合物与N-溴邻苯二甲酰亚胺的P–N交叉偶联。氮源可能来自内部N-溴邻苯二甲酰亚胺本身(自消灭试剂)或外部邻苯二甲酰亚胺衍生物(具有NBS-DBU活化系统)。该反应具有宽泛的磷化合物范围,包括H-次膦酸酯,H-膦酸酯和H-膦氧化物,并且反应时间短(少于10分钟)。该方法为在温和条件下具有生物学重要性的氨基磷酸酯提供了一种简单,方便,有效和绿色的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号