当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Application of N‐Protected 3‐Vinylindoles in Chiral Phosphoric Acid‐Catalyzed [3+2] Cyclization with 3‐Indolylmethanols: Monoactivation of the Catalyst to Vinyliminium
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-06-02 , DOI: 10.1002/adsc.201501175 Tao Fan 1 , Hong‐Hao Zhang 1 , Can Li 1 , Yang Shen 1 , Feng Shi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-06-02 , DOI: 10.1002/adsc.201501175 Tao Fan 1 , Hong‐Hao Zhang 1 , Can Li 1 , Yang Shen 1 , Feng Shi 1
Affiliation
|
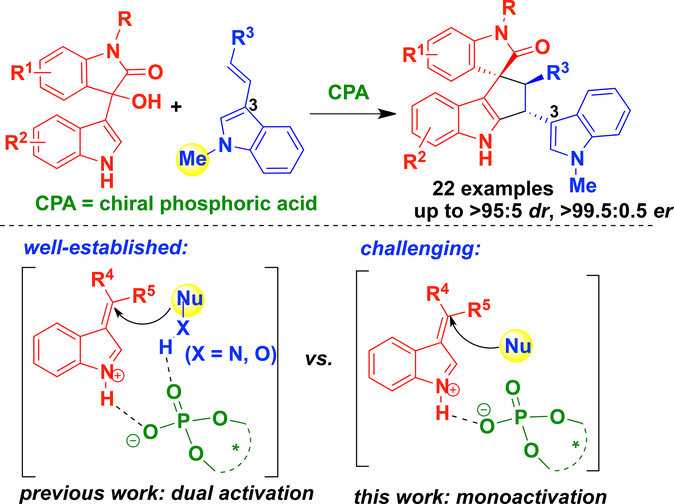
A highly enantioselective [3+2] cyclization of 3‐vinylindoles with isatin‐derived 3‐indolylmethanols has been established in the presence of chiral phosphoric acid, which constructed the enantioenriched cyclopenta[b]indole scaffold linking a 3‐vinylindole moiety in generally good yields, considerable diastereoselectivities and high enantioselectivities (up to 93%, >95:5 dr, >99.5:0.5 er). More importantly, this reaction utilized N‐protected 3‐vinylindoles as competent reactants, which confronted the challenge in realizing a successful monoactivation mode of chiral phosphoric acid to vinyliminium in 3‐indolylmethanol‐involved enantioselective transformations. Besides, this protocol also settled the challenge in realizing a highly enantioselective [3+2] cyclization of 3‐vinylindoles with 3‐indolylmethanols, which would serve as a useful strategy for constructing an enantioenriched cyclopenta[b]indole scaffold linking a 3‐vinylindole moiety.
中文翻译:

N保护的3-Vinylindoles在3-吲哚基甲醇催化手性磷酸催化的[3 + 2]环化中的应用:催化剂对乙烯基亚胺的单活化
在存在手性磷酸的情况下,已建立了由靛红衍生的3-吲哚基甲醇对3-乙烯基吲哚的高度对映选择性[3 + 2]环化反应,该化合物构建了对映体富集的环戊达[ b ]吲哚骨架,该骨架通常将3-乙烯基吲哚部分连接起来。收率,相当的非对映选择性和高对映选择性(高达93%,> 95:5 dr,> 99.5:0.5 er)。更重要的是,该反应利用了N-保护的3-乙烯基吲哚作为有效的反应物,这在实现由3-吲哚基甲醇参与的对映选择性转化中,成功地将手性磷酸单活化成乙烯基亚胺时面临着挑战。此外,该协议还解决了用3-吲哚基甲醇实现3-乙烯基吲哚的高度对映选择性[3 + 2]环化的挑战,这将成为构建连接3-乙烯基吲哚的对映体富集的环戊达[ b ]吲哚支架的有用策略。部分。
更新日期:2016-06-02
中文翻译:

N保护的3-Vinylindoles在3-吲哚基甲醇催化手性磷酸催化的[3 + 2]环化中的应用:催化剂对乙烯基亚胺的单活化
在存在手性磷酸的情况下,已建立了由靛红衍生的3-吲哚基甲醇对3-乙烯基吲哚的高度对映选择性[3 + 2]环化反应,该化合物构建了对映体富集的环戊达[ b ]吲哚骨架,该骨架通常将3-乙烯基吲哚部分连接起来。收率,相当的非对映选择性和高对映选择性(高达93%,> 95:5 dr,> 99.5:0.5 er)。更重要的是,该反应利用了N-保护的3-乙烯基吲哚作为有效的反应物,这在实现由3-吲哚基甲醇参与的对映选择性转化中,成功地将手性磷酸单活化成乙烯基亚胺时面临着挑战。此外,该协议还解决了用3-吲哚基甲醇实现3-乙烯基吲哚的高度对映选择性[3 + 2]环化的挑战,这将成为构建连接3-乙烯基吲哚的对映体富集的环戊达[ b ]吲哚支架的有用策略。部分。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号