当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation, Crystal Structure, and Luminescence Properties of Zeolite LTA Containing Extraframework Tantalum(V), Tantalum(II), Thallium(I), and Chloride
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-05-31 00:00:00 , DOI: 10.1021/acs.jpcc.6b03943 Hyeon Seung Lim 1 , Joon Young Kim 1 , Nam Ho Heo 1 , Karl Seff 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-05-31 00:00:00 , DOI: 10.1021/acs.jpcc.6b03943 Hyeon Seung Lim 1 , Joon Young Kim 1 , Nam Ho Heo 1 , Karl Seff 2
Affiliation
|
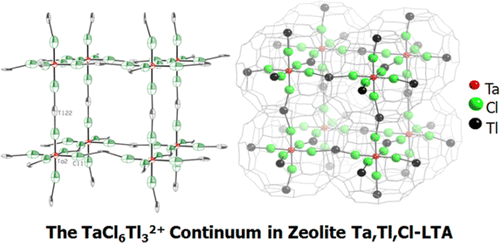
For the first time pentapositive cations have been introduced into a zeolite at extraframework positions. Their presence was confirmed by single-crystal crystallography using synchrotron X-radiation and energy-dispersive X-ray analysis. It was prepared by the thallous ion exchange (TIE) method: fully dehydrated Tl12-LTA was treated with 3.0 × 104 Pa of TaCl5(g) at 473 K. The crystal structure of the product, |Ta2+0.123(TaCl6Tl3)2+0.246Tl+9.84|[Si13.4Al10.6O48]-LTA, was refined in the space group Pm3̅m (a = 12.099(1) Å) with all unique data to the final error index R1 = 0.044 for the 639 reflections for which Fo > 4σ(Fo). TIE did not occur. Instead some TaCl5 molecules decomposed to TaCl2 and Cl2(g), and TaCl2 reacted with additional TaCl5 molecules to form Ta2+ and TaCl6–. Octahedral TaCl6– ions center about 24.6% of the large cavities. Each Cl– ion bonds to a Tl+ ion in the plane of an 8-ring to form a TaCl6Tl32+ continuum in the near-surface volume of the crystal. The Ta2+ ions, 0.123(3) per unit cell, lie opposite 6-rings in the large cavities. All Tl+ ions occupy well-established cation positions near 6- and 8-rings. The photoluminescence spectrum of this material is a broad emission band between 350 and 500 nm, peaking at 410 nm.
中文翻译:

含骨架(V),钽(II),T(I)和氯化物的沸石LTA的制备,晶体结构和发光性能
五元阳离子正第一次被引入到骨架外位置的沸石中。通过使用同步加速器X射线和能量色散X射线分析的单晶晶体学证实了它们的存在。它是通过th离子交换(TIE)方法制备的:在473 K下用3.0×10 4 Pa的TaCl 5(g)处理完全脱水的Tl 12 -LTA 。产物的晶体结构| Ta 2+ 0.123( TaCl 6 Tl 3)2+ 0.246 Tl + 9.84 | [Si 13.4 Al 10.6 O 48] -LTA,空间群中进行精制PM 3米(一个= 12.099(1)a)用所有唯一数据到最终误差指数- [R 1 = 0.044的639个反射为其˚F ø >4σ(˚F Ò)。没有发生TIE。相反,一些TaCl 5分子分解为TaCl 2和Cl 2(g),而TaCl 2与另外的TaCl 5分子反应形成Ta 2+和TaCl 6 –。八面体TaCl 6 –离子居中于大腔的约24.6%。各自为Cl -离子键合到一个铊+在8环平面离子以形成TACL 6铊3 2+在晶体的近表面体积连续统一体。Ta 2+离子,每晶胞0.123(3),在大腔中与6环相对。所有的Tl +离子都占据6环和8环附近公认的阳离子位置。该材料的光致发光光谱是在350至500 nm之间的宽发射带,在410 nm处达到峰值。
更新日期:2016-05-31
中文翻译:

含骨架(V),钽(II),T(I)和氯化物的沸石LTA的制备,晶体结构和发光性能
五元阳离子正第一次被引入到骨架外位置的沸石中。通过使用同步加速器X射线和能量色散X射线分析的单晶晶体学证实了它们的存在。它是通过th离子交换(TIE)方法制备的:在473 K下用3.0×10 4 Pa的TaCl 5(g)处理完全脱水的Tl 12 -LTA 。产物的晶体结构| Ta 2+ 0.123( TaCl 6 Tl 3)2+ 0.246 Tl + 9.84 | [Si 13.4 Al 10.6 O 48] -LTA,空间群中进行精制PM 3米(一个= 12.099(1)a)用所有唯一数据到最终误差指数- [R 1 = 0.044的639个反射为其˚F ø >4σ(˚F Ò)。没有发生TIE。相反,一些TaCl 5分子分解为TaCl 2和Cl 2(g),而TaCl 2与另外的TaCl 5分子反应形成Ta 2+和TaCl 6 –。八面体TaCl 6 –离子居中于大腔的约24.6%。各自为Cl -离子键合到一个铊+在8环平面离子以形成TACL 6铊3 2+在晶体的近表面体积连续统一体。Ta 2+离子,每晶胞0.123(3),在大腔中与6环相对。所有的Tl +离子都占据6环和8环附近公认的阳离子位置。该材料的光致发光光谱是在350至500 nm之间的宽发射带,在410 nm处达到峰值。































 京公网安备 11010802027423号
京公网安备 11010802027423号