当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rifamycin Biosynthetic Congeners: Isolation and Total Synthesis of Rifsaliniketal and Total Synthesis of Salinisporamycin and Saliniketals A and B
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-05-27 , DOI: 10.1021/jacs.6b03248 Yu Feng 1 , Jun Liu 1 , Yazmin P. Carrasco 1 , John B. MacMillan 1 , Jef K. De Brabander 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-05-27 , DOI: 10.1021/jacs.6b03248 Yu Feng 1 , Jun Liu 1 , Yazmin P. Carrasco 1 , John B. MacMillan 1 , Jef K. De Brabander 1
Affiliation
|
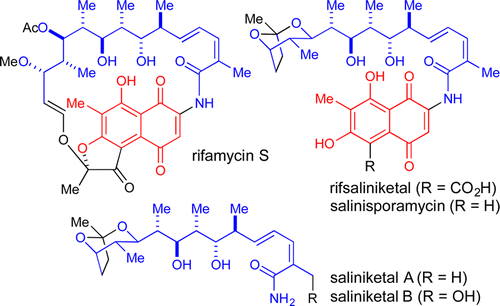
We describe the isolation, structure elucidation, and total synthesis of the novel marine natural product rifsaliniketal and the total synthesis of the structurally related variants salinisporamycin and saliniketals A and B. Rifsaliniketal was previously proposed, but not observed, as a diverted metabolite from a biosynthetic precursor to rifamycin S. Decarboxylation of rifamycin provides salinisporamycin, which upon truncation with loss of the naphthoquinone ring leads to saliniketals. Our synthetic strategy hinged upon a Pt(II)-catalyzed cycloisomerization of an alkynediol to set the dioxabicyclo[3.2.1]octane ring system and a fragmentation of an intermediate dihydropyranone to forge a stereochemically defined (E,Z)-dienamide unit. Multiple routes were explored to assemble fragments with high stereocontrol, an exercise that provided additional insights into acyclic stereocontrol during stereochemically complex fragment-assembly processes. The resulting 11-14 step synthesis of saliniketals then enabled us to explore strategies for the synthesis and coupling of highly substituted naphthoquinones or the corresponding naphthalene fragments. Whereas direct coupling with naphthoquinone fragments proved unsuccessful, both amidation and C-N bond formation tactics with the more electron-rich naphthalene congeners provided an efficient means to complete the first total synthesis of rifsaliniketal and salinisporamycin.
中文翻译:

利福霉素生物合成同源物:利福沙林酮的分离和全合成以及盐孢霉素和盐缩酮 A 和 B 的全合成
我们描述了新型海洋天然产物利夫沙林酮的分离、结构解析和全合成,以及结构相关变体盐孢霉素和盐酮 A 和 B 的全合成。 利夫沙林酮先前被提出,但未观察到,作为来自生物合成的转移代谢物利福霉素 S 的前体。利福霉素脱羧产生盐孢霉素,它在截断并失去萘醌环后产生盐缩酮。我们的合成策略取决于 Pt(II) 催化的炔二醇环异构化以设置二氧杂双环 [3.2.1] 辛烷环系统和中间体二氢吡喃酮的碎片化以形成立体化学定义的 (E,Z)-二烯酰胺单元。探索了多种途径来组装具有高度立体控制的片段,在立体化学复杂的片段组装过程中提供了对无环立体控制的额外见解的练习。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。
更新日期:2016-05-27
中文翻译:

利福霉素生物合成同源物:利福沙林酮的分离和全合成以及盐孢霉素和盐缩酮 A 和 B 的全合成
我们描述了新型海洋天然产物利夫沙林酮的分离、结构解析和全合成,以及结构相关变体盐孢霉素和盐酮 A 和 B 的全合成。 利夫沙林酮先前被提出,但未观察到,作为来自生物合成的转移代谢物利福霉素 S 的前体。利福霉素脱羧产生盐孢霉素,它在截断并失去萘醌环后产生盐缩酮。我们的合成策略取决于 Pt(II) 催化的炔二醇环异构化以设置二氧杂双环 [3.2.1] 辛烷环系统和中间体二氢吡喃酮的碎片化以形成立体化学定义的 (E,Z)-二烯酰胺单元。探索了多种途径来组装具有高度立体控制的片段,在立体化学复杂的片段组装过程中提供了对无环立体控制的额外见解的练习。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。由此产生的盐缩酮的 11-14 步合成使我们能够探索合成和偶联高度取代的萘醌或相应的萘片段的策略。尽管与萘醌片段的直接偶联被证明是不成功的,但酰胺化和 CN 键形成策略与更富电子的萘同系物提供了一种有效的手段来完成利沙林酮和盐孢霉素的首次全合成。















































 京公网安备 11010802027423号
京公网安备 11010802027423号