当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
X-ray Crystallographic Structure and Solution Behavior of an Antiparallel Coiled-Coil Hexamer Formed by de Novo Peptides
Biochemistry ( IF 2.9 ) Pub Date : 2016-05-27 00:00:00 , DOI: 10.1021/acs.biochem.6b00201 Ryan K. Spencer 1 , Allon I. Hochbaum 1
Biochemistry ( IF 2.9 ) Pub Date : 2016-05-27 00:00:00 , DOI: 10.1021/acs.biochem.6b00201 Ryan K. Spencer 1 , Allon I. Hochbaum 1
Affiliation
|
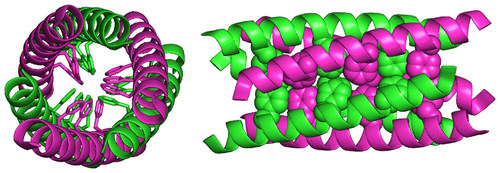
The self-assembly of peptides and proteins into higher-ordered structures is encoded in the amino acid sequence of each peptide or protein. Understanding the relationship among the amino acid sequence, the assembly dynamics, and the structure of well-defined peptide oligomers expands the synthetic toolbox for these structures. Here, we present the X-ray crystallographic structure and solution behavior of de novo peptides that form antiparallel coiled-coil hexamers (ACC-Hex) by an interaction motif neither found in nature nor predicted by existing peptide design software. The 1.70 Å X-ray crystallographic structure of peptide 1a shows six α-helices associating in an antiparallel arrangement around a central axis comprising hydrophobic and aromatic residues. Size-exclusion chromatography studies suggest that peptides 1 form stable oligomers in solution, and circular dichroism experiments show that peptides 1 are stable to relatively high temperatures. Small-angle X-ray scattering studies of the solution behavior of peptide 1a indicate an equilibrium of dimers, hexamers, and larger aggregates in solution. The structures presented here represent a new motif of biomolecular self-assembly not previously observed for de novo peptides and suggest supramolecular design principles for material scaffolds based on coiled-coil motifs containing aromatic residues.
中文翻译:

de Novo肽形成的反平行卷曲螺旋六聚体的X射线晶体学结构和溶液行为
肽和蛋白质自组装成高阶结构的过程会在每种肽或蛋白质的氨基酸序列中进行编码。了解氨基酸序列,装配动力学和明确定义的肽寡聚物的结构之间的关系,将扩展这些结构的合成工具箱。在这里,我们通过自然界中既没有发现也没有现有肽设计软件预测的相互作用基序,形成反平行卷曲螺旋六聚体(ACC-Hex)的从头肽的X射线晶体学结构和溶液行为。肽1a的1.70ÅX射线晶体结构图6显示了围绕中心轴以反平行排列缔合的六个α-螺旋,其包含疏水和芳族残基。尺寸排阻色谱研究表明,肽1在溶液中形成稳定的低聚物,而圆二色性实验表明,肽1在相对较高的温度下是稳定的。肽1a溶液行为的小角X射线散射研究表明溶液中二聚体,六聚体和较大聚集体的平衡。本文介绍的结构代表了以前从未从头发现过的生物分子自组装的新基序,并提出了基于包含芳香族残基的卷曲螺旋基序的材料支架的超分子设计原理。
更新日期:2016-05-27
中文翻译:

de Novo肽形成的反平行卷曲螺旋六聚体的X射线晶体学结构和溶液行为
肽和蛋白质自组装成高阶结构的过程会在每种肽或蛋白质的氨基酸序列中进行编码。了解氨基酸序列,装配动力学和明确定义的肽寡聚物的结构之间的关系,将扩展这些结构的合成工具箱。在这里,我们通过自然界中既没有发现也没有现有肽设计软件预测的相互作用基序,形成反平行卷曲螺旋六聚体(ACC-Hex)的从头肽的X射线晶体学结构和溶液行为。肽1a的1.70ÅX射线晶体结构图6显示了围绕中心轴以反平行排列缔合的六个α-螺旋,其包含疏水和芳族残基。尺寸排阻色谱研究表明,肽1在溶液中形成稳定的低聚物,而圆二色性实验表明,肽1在相对较高的温度下是稳定的。肽1a溶液行为的小角X射线散射研究表明溶液中二聚体,六聚体和较大聚集体的平衡。本文介绍的结构代表了以前从未从头发现过的生物分子自组装的新基序,并提出了基于包含芳香族残基的卷曲螺旋基序的材料支架的超分子设计原理。







































 京公网安备 11010802027423号
京公网安备 11010802027423号