当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Essential structural elements in tRNA(Pro) for EF-P-mediated alleviation of translation stalling.
Nature Communications ( IF 14.7 ) Pub Date : 2016-05-24 , DOI: 10.1038/ncomms11657 Takayuki Katoh , Ingo Wohlgemuth , Masanobu Nagano , Marina V. Rodnina , Hiroaki Suga
Nature Communications ( IF 14.7 ) Pub Date : 2016-05-24 , DOI: 10.1038/ncomms11657 Takayuki Katoh , Ingo Wohlgemuth , Masanobu Nagano , Marina V. Rodnina , Hiroaki Suga
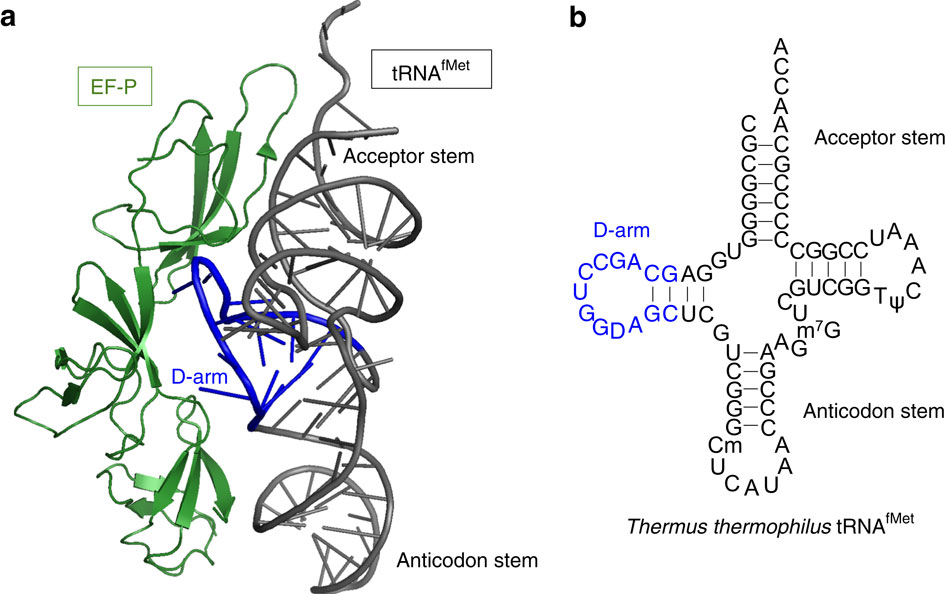
|
The ribosome stalls on translation of polyproline sequences due to inefficient peptide bond formation between consecutive prolines. The translation factor EF-P is able to alleviate this stalling by accelerating Pro-Pro formation. However, the mechanism by which EF-P recognizes the stalled complexes and accelerates peptide bond formation is not known. Here, we use genetic code reprogramming through a flexible in-vitro translation (FIT) system to investigate how mutations in tRNA(Pro) affect EF-P function. We show that the 9-nt D-loop closed by the stable D-stem sequence in tRNA(Pro) is a crucial recognition determinant for EF-P. Such D-arm structures are shared only among the tRNA(Pro) isoacceptors and tRNA(fMet) in Escherichia coli, and the D-arm of tRNA(fMet) is essential for EF-P-induced acceleration of fMet-puromycin formation. Thus, the activity of EF-P is controlled by recognition elements in the tRNA D-arm.
中文翻译:

tRNA(Pro)中用于EF-P介导的翻译停滞缓解的基本结构元件。
由于连续的脯氨酸之间的肽键形成效率低下,核糖体在多脯氨酸序列的翻译中停滞。转化因子EF-P可以通过加速Pro-Pro的形成来减轻这种拖延。但是,EF-P识别失速复合物并加速肽键形成的机理尚不清楚。在这里,我们通过灵活的体外翻译(FIT)系统使用遗传密码重新编程来研究tRNA(Pro)中的突变如何影响EF-P功能。我们显示,tRNA(Pro)中稳定的D-茎序列封闭的9-nt D环是EF-P的关键识别决定因素。此类D臂结构仅在大肠杆菌中的tRNA(Pro)同种受体和tRNA(fMet)之间共享,而tRNA(fMet)的D臂对于EF-P诱导的fMet-嘌呤霉素形成的加速至关重要。因此,
更新日期:2016-05-27
中文翻译:

tRNA(Pro)中用于EF-P介导的翻译停滞缓解的基本结构元件。
由于连续的脯氨酸之间的肽键形成效率低下,核糖体在多脯氨酸序列的翻译中停滞。转化因子EF-P可以通过加速Pro-Pro的形成来减轻这种拖延。但是,EF-P识别失速复合物并加速肽键形成的机理尚不清楚。在这里,我们通过灵活的体外翻译(FIT)系统使用遗传密码重新编程来研究tRNA(Pro)中的突变如何影响EF-P功能。我们显示,tRNA(Pro)中稳定的D-茎序列封闭的9-nt D环是EF-P的关键识别决定因素。此类D臂结构仅在大肠杆菌中的tRNA(Pro)同种受体和tRNA(fMet)之间共享,而tRNA(fMet)的D臂对于EF-P诱导的fMet-嘌呤霉素形成的加速至关重要。因此,






























 京公网安备 11010802027423号
京公网安备 11010802027423号