当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Mediated Meerwein-Type Reaction: A Radical Cascade Arylation/Aryl Migration/Desulfonylation of Conjugated Alkenes
Organic Letters ( IF 4.9 ) Pub Date : 2016-05-24 00:00:00 , DOI: 10.1021/acs.orglett.6b01041 Zhangqin Ni 1 , Xin Huang 1 , Yuanjiang Pan 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-05-24 00:00:00 , DOI: 10.1021/acs.orglett.6b01041 Zhangqin Ni 1 , Xin Huang 1 , Yuanjiang Pan 1
Affiliation
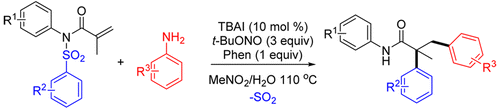
|
A metal-free cascade arylation/aryl migration/desulfonylation of N-phenyl-N-(phenylsulfonyl)methacrylamide is described. The in situ generated diazonium salts from anilines and t-BuONO are used as aryl precursors. This process provides an efficient strategy for the synthesis of α-all-carbon quaternary stereocenters amides. A radical mechanism was proposed for this transformation.
中文翻译:

无金属介导的Meerwein型反应:共轭烯烃的自由基级联芳基化/芳基迁移/脱磺酰化
描述了N-苯基-N-(苯基磺酰基)甲基丙烯酰胺的无金属级联芳基化/芳基迁移/去磺酰化。由苯胺和叔-BuONO原位产生的重氮盐用作芳基前体。该方法为合成α-全碳四元立体中心酰胺提供了有效的策略。为这种转变提出了一种激进的机制。
更新日期:2016-05-24
中文翻译:

无金属介导的Meerwein型反应:共轭烯烃的自由基级联芳基化/芳基迁移/脱磺酰化
描述了N-苯基-N-(苯基磺酰基)甲基丙烯酰胺的无金属级联芳基化/芳基迁移/去磺酰化。由苯胺和叔-BuONO原位产生的重氮盐用作芳基前体。该方法为合成α-全碳四元立体中心酰胺提供了有效的策略。为这种转变提出了一种激进的机制。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号