当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Sulfur Transfer Across Protein–Protein Interfaces: The Cysteine Desulfurase Model System
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-05-23 00:00:00 , DOI: 10.1021/acscatal.6b00360
Francisco J. Fernández 1 , Ana Ardá 1, 2 , Miguel López-Estepa 1 , Juan Aranda 3 , Esther Peña-Soler 1 , Fernando Garces 4 , Adam Round 5, 6 , Ramón Campos-Olivas 7 , Marta Bruix 8 , Miquel Coll 9, 10 , Iñaki Tuñón 3 , Jesús Jiménez-Barbero 1, 2, 11, 12 , M. Cristina Vega 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-05-23 00:00:00 , DOI: 10.1021/acscatal.6b00360
Francisco J. Fernández 1 , Ana Ardá 1, 2 , Miguel López-Estepa 1 , Juan Aranda 3 , Esther Peña-Soler 1 , Fernando Garces 4 , Adam Round 5, 6 , Ramón Campos-Olivas 7 , Marta Bruix 8 , Miquel Coll 9, 10 , Iñaki Tuñón 3 , Jesús Jiménez-Barbero 1, 2, 11, 12 , M. Cristina Vega 1
Affiliation
|
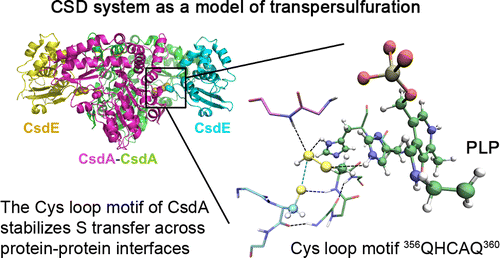
CsdA cysteine desulfurase (the sulfur donor) and the CsdE sulfur acceptor are involved in biological sulfur trafficking and in iron–sulfur cluster assembly in the model bacterium Escherichia coli. CsdA and CsdE form a stable complex through a polar interface that includes CsdA Cys328 and CsdE Cys61, the two residues known to be involved in the sulfur transfer reaction. Although mechanisms for the transfer of a sulfur moiety across protein–protein interfaces have been proposed based on the IscS–IscU and IscS–TusA structures, the flexibility of the catalytic cysteine loops involved has precluded a high resolution view of the active-site geometry and chemical environment for sulfur transfer. Here, we have used a combination of X-ray crystallography, solution NMR and SAXS, isothermal calorimetry, and computational chemistry methods to unravel how CsdA provides a specific recognition platform for CsdE and how their complex organizes a composite functional reaction environment. The X-ray structures of persulfurated (CsdA)2 and persulfurated (CsdA–CsdE)2 complexes reveal the crucial roles of the conserved active-site cysteine loop and additional catalytic residues in supporting the transpersulfuration reaction. A mechanistic view of sulfur transfer across protein–protein interfaces that underpins the requirement for the conserved cysteine loop to provide electrostatic stabilization for the in-transfer sulfur atom emerges from the analysis of the persulfurated (CsdA–CsdE)2 complex structure.
中文翻译:

跨蛋白质-蛋白质界面的硫转移机理:半胱氨酸脱硫酶模型系统
CsdA半胱氨酸脱硫酶(硫供体)和CsdE硫受体参与了模型细菌大肠杆菌中的生物硫运输和铁硫簇组装。。CsdA和CsdE通过极性界面形成稳定的复合物,该界面包括CsdA Cys328和CsdE Cys61,这两个已知参与硫转移反应的残基。尽管已经基于IscS–IscU和IscS–TusA结构提出了跨蛋白质-蛋白质界面转移硫部分的机制,但是所涉及的催化半胱氨酸环的灵活性已排除了高分辨率的活性位点几何结构和硫转移的化学环境。在这里,我们结合使用了X射线晶体学,溶液NMR和SAXS,等温量热法和计算化学方法,以阐明CsdA如何为CsdE提供特定的识别平台以及它们的配合物如何构成复合功能反应环境。过硫酸盐(CsdA)的X射线结构2和过硫酸化(CsdA–CsdE)2配合物揭示了保守的活性位点半胱氨酸环和其他催化残基在支持反全硫反应中的关键作用。从过硫酸化(CsdA–CsdE)2复杂结构的分析中得出了一种跨蛋白质-蛋白质界面进行硫转移的机制观点,该观点支持了保守的半胱氨酸环为转移中的硫原子提供静电稳定性的要求。
更新日期:2016-05-23
中文翻译:

跨蛋白质-蛋白质界面的硫转移机理:半胱氨酸脱硫酶模型系统
CsdA半胱氨酸脱硫酶(硫供体)和CsdE硫受体参与了模型细菌大肠杆菌中的生物硫运输和铁硫簇组装。。CsdA和CsdE通过极性界面形成稳定的复合物,该界面包括CsdA Cys328和CsdE Cys61,这两个已知参与硫转移反应的残基。尽管已经基于IscS–IscU和IscS–TusA结构提出了跨蛋白质-蛋白质界面转移硫部分的机制,但是所涉及的催化半胱氨酸环的灵活性已排除了高分辨率的活性位点几何结构和硫转移的化学环境。在这里,我们结合使用了X射线晶体学,溶液NMR和SAXS,等温量热法和计算化学方法,以阐明CsdA如何为CsdE提供特定的识别平台以及它们的配合物如何构成复合功能反应环境。过硫酸盐(CsdA)的X射线结构2和过硫酸化(CsdA–CsdE)2配合物揭示了保守的活性位点半胱氨酸环和其他催化残基在支持反全硫反应中的关键作用。从过硫酸化(CsdA–CsdE)2复杂结构的分析中得出了一种跨蛋白质-蛋白质界面进行硫转移的机制观点,该观点支持了保守的半胱氨酸环为转移中的硫原子提供静电稳定性的要求。

































 京公网安备 11010802027423号
京公网安备 11010802027423号