当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of 1-Methyl-1,4-dihydroindeno[1,2-c]pyrazole Analogues as Potential Anticancer Agents Targeting Tubulin Colchicine Binding Site
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-05-20 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00071 Yan-Na Liu 1 , Jing-Jing Wang 1 , Ya-Ting Ji 1 , Guo-Dong Zhao 1 , Long-Qian Tang 1 , Cheng-Mei Zhang 1 , Xiu-Li Guo 1 , Zhao-Peng Liu 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-05-20 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00071 Yan-Na Liu 1 , Jing-Jing Wang 1 , Ya-Ting Ji 1 , Guo-Dong Zhao 1 , Long-Qian Tang 1 , Cheng-Mei Zhang 1 , Xiu-Li Guo 1 , Zhao-Peng Liu 1
Affiliation
|
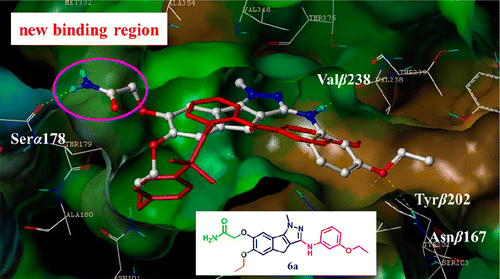
By targeting a new binding region at the interface between αβ-tubulin heterodimers at the colchicine binding site, we designed a series of 7-substituted 1-methyl-1,4-dihydroindeno[1,2-c]pyrazoles as potential tubulin polymerization inhibitors. Among the compounds synthesized, 2-(6-ethoxy-3-(3-ethoxyphenylamino)-1-methyl-1,4-dihydroindeno[1,2-c]pyrazol-7-yloxy)acetamide 6a and 2-(6-ethoxy-3-(3-ethoxyphenylamino)-1-methyl-1,4-dihydroindeno[1,2-c]pyrazol-7-yloxy)-N-hydroxyacetamide 6n showed noteworthy low nanomolar potency against HepG2, Hela, PC3, and MCF-7 cancer cell lines. In mechanism studies, 6a inhibited tubulin polymerization and disorganized microtubule in A549 cells by binding to tubulin colchicine binding site. 6a arrested A549 cells in G2/M phase that was related to the alterations in the expression of cyclin B1 and p-cdc2. 6a induced A549 cells apoptosis through the activation of caspase-3 and PARP. In addition, 6a inhibited capillary tube formation in a concentration-dependent manner. In nonsmall cell lung cancer xenografts mouse model, 6a suppressed tumor growth by 59.1% at a dose of 50 mg/kg (ip) without obvious toxicity, indicating its in vivo potential as anticancer agent.
中文翻译:

设计,合成和生物评价1-甲基-1,4-二氢茚并[1,2- c ]吡唑类似物作为潜在的靶向微管蛋白秋水仙碱结合位点的抗癌药。
通过在秋水仙碱结合位点的αβ-微管蛋白异二聚体之间的界面处靶向新的结合区域,我们设计了一系列7-取代的1-甲基-1,4-二氢茚并[1,2- c ]吡唑类作为潜在的微管蛋白聚合抑制剂。在合成的化合物中,2-(6-乙氧基-3-(3-乙氧基苯基氨基)-1-甲基-1,4-二氢茚并[1,2 - c ]吡唑-7-酰氧基)乙酰胺6a和2-(6-乙氧基-3-(3-乙氧基苯基氨基)-1-甲基-1,4-二氢茚并[1,2- c ]吡唑-7-酰氧基)-N-羟基乙酰胺6n对HepG2,Hela,PC3和MCF-7癌细胞系。在机理研究中,6a通过与微管蛋白秋水仙碱结合位点结合,抑制A549细胞中的微管蛋白聚合和微管紊乱。6a在G2 / M期阻滞了A549细胞,这与细胞周期蛋白B1和p-cdc2表达的改变有关。6a通过激活caspase-3和PARP诱导A549细胞凋亡。另外,6a以浓度依赖的方式抑制毛细管的形成。在非小细胞肺癌异种移植小鼠模型中,6a以50 mg / kg(ip)的剂量将肿瘤生长抑制了59.1%,而没有明显的毒性,表明其在体内具有作为抗癌剂的潜力。
更新日期:2016-05-20
中文翻译:

设计,合成和生物评价1-甲基-1,4-二氢茚并[1,2- c ]吡唑类似物作为潜在的靶向微管蛋白秋水仙碱结合位点的抗癌药。
通过在秋水仙碱结合位点的αβ-微管蛋白异二聚体之间的界面处靶向新的结合区域,我们设计了一系列7-取代的1-甲基-1,4-二氢茚并[1,2- c ]吡唑类作为潜在的微管蛋白聚合抑制剂。在合成的化合物中,2-(6-乙氧基-3-(3-乙氧基苯基氨基)-1-甲基-1,4-二氢茚并[1,2 - c ]吡唑-7-酰氧基)乙酰胺6a和2-(6-乙氧基-3-(3-乙氧基苯基氨基)-1-甲基-1,4-二氢茚并[1,2- c ]吡唑-7-酰氧基)-N-羟基乙酰胺6n对HepG2,Hela,PC3和MCF-7癌细胞系。在机理研究中,6a通过与微管蛋白秋水仙碱结合位点结合,抑制A549细胞中的微管蛋白聚合和微管紊乱。6a在G2 / M期阻滞了A549细胞,这与细胞周期蛋白B1和p-cdc2表达的改变有关。6a通过激活caspase-3和PARP诱导A549细胞凋亡。另外,6a以浓度依赖的方式抑制毛细管的形成。在非小细胞肺癌异种移植小鼠模型中,6a以50 mg / kg(ip)的剂量将肿瘤生长抑制了59.1%,而没有明显的毒性,表明其在体内具有作为抗癌剂的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号