当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of Hypoxia in Solid Tumor Microenvironment with MnO2 Nanoparticles to Enhance Photodynamic Therapy
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2016-05-20 , DOI: 10.1002/adfm.201600676 Wenwen Zhu 1 , Ziliang Dong 1 , Tingting Fu 2 , Jingjing Liu 1 , Qian Chen 1 , Yonggang Li 2 , Ran Zhu 3 , Ligeng Xu 1 , Zhuang Liu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2016-05-20 , DOI: 10.1002/adfm.201600676 Wenwen Zhu 1 , Ziliang Dong 1 , Tingting Fu 2 , Jingjing Liu 1 , Qian Chen 1 , Yonggang Li 2 , Ran Zhu 3 , Ligeng Xu 1 , Zhuang Liu 1
Affiliation
|
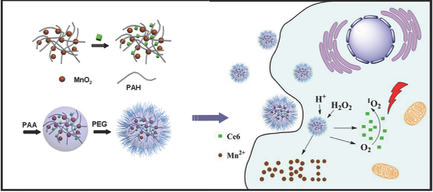
Hypoxia not only promotes tumor metastasis but also strengthens tumor resistance to therapies that demand the involvement of oxygen, such as radiation therapy and photodynamic therapy (PDT). Herein, taking advantage of the high reactivity of manganese dioxide (MnO2) nanoparticles toward endogenous hydrogen peroxide (H2O2) within the tumor microenvironment to generate O2, multifunctional chlorine e6 (Ce6) loaded MnO2 nanoparticles with surface polyethylene glycol (PEG) modification (Ce6@MnO2‐PEG) are formulated to achieve enhanced tumor‐specific PDT. In vitro studies under an oxygen‐deficient atmosphere uncover that Ce6@MnO2‐PEG nanoparticles could effectively enhance the efficacy of light‐induced PDT due to the increased intracellular O2 level benefited from the reaction between MnO2 and H2O2, the latter of which is produced by cancer cells under the hypoxic condition. Owing to the efficient tumor homing of Ce6@MnO2‐PEG nanoparticles upon intravenous injection as revealed by T1‐weighted magnetic resonance imaging, the intratumoral hypoxia is alleviated to a great extent. Thus, in vivo PDT with Ce6@MnO2‐PEG nanoparticles even at a largely reduced dose offers remarkably improved therapeutic efficacy in inhibiting tumor growth compared to free Ce6. The results highlight the promise of modulating unfavorable tumor microenvironment with nanotechnology to overcome current limitations of cancer therapies.
中文翻译:

MnO2纳米粒子在固体肿瘤微环境中的缺氧调节以增强光动力疗法。
缺氧不仅促进肿瘤转移,而且增强了肿瘤对需要氧气介入的疗法的抵抗力,例如放射疗法和光动力疗法(PDT)。在此,利用肿瘤微环境中二氧化锰(MnO 2)纳米粒子对内源性过氧化氢(H 2 O 2)的高反应性来生成O 2,负载多功能氯e6(Ce6)的MnO 2纳米粒子与表面聚乙二醇(配制了PEG)修饰(Ce6 @ MnO 2 -PEG)以实现增强的肿瘤特异性PDT。在缺氧气氛下的体外研究发现Ce6 @ MnO 2‐PEG纳米颗粒可以有效地增强光诱导PDT的功效,这是由于MnO 2和H 2 O 2之间的反应受益于细胞内O 2含量的增加,后者是由缺氧条件下的癌细胞产生的。T1加权磁共振成像显示,由于静脉注射后Ce6 @ MnO 2 -PEG纳米颗粒具有高效的肿瘤归巢能力,因此可以大大缓解肿瘤内的缺氧。因此,体内含Ce6 @ MnO 2的PDT‐PEG纳米粒子与游离Ce6相比,即使在大幅降低剂量的情况下,在抑制肿瘤生长方面也能显着提高治疗效果。结果突出了用纳米技术调节不利的肿瘤微环境以克服目前癌症治疗的局限性的希望。
更新日期:2016-05-20
中文翻译:

MnO2纳米粒子在固体肿瘤微环境中的缺氧调节以增强光动力疗法。
缺氧不仅促进肿瘤转移,而且增强了肿瘤对需要氧气介入的疗法的抵抗力,例如放射疗法和光动力疗法(PDT)。在此,利用肿瘤微环境中二氧化锰(MnO 2)纳米粒子对内源性过氧化氢(H 2 O 2)的高反应性来生成O 2,负载多功能氯e6(Ce6)的MnO 2纳米粒子与表面聚乙二醇(配制了PEG)修饰(Ce6 @ MnO 2 -PEG)以实现增强的肿瘤特异性PDT。在缺氧气氛下的体外研究发现Ce6 @ MnO 2‐PEG纳米颗粒可以有效地增强光诱导PDT的功效,这是由于MnO 2和H 2 O 2之间的反应受益于细胞内O 2含量的增加,后者是由缺氧条件下的癌细胞产生的。T1加权磁共振成像显示,由于静脉注射后Ce6 @ MnO 2 -PEG纳米颗粒具有高效的肿瘤归巢能力,因此可以大大缓解肿瘤内的缺氧。因此,体内含Ce6 @ MnO 2的PDT‐PEG纳米粒子与游离Ce6相比,即使在大幅降低剂量的情况下,在抑制肿瘤生长方面也能显着提高治疗效果。结果突出了用纳米技术调节不利的肿瘤微环境以克服目前癌症治疗的局限性的希望。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号