当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Discovery and Characterization of the α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Potentiator N-{(3S,4S)-4-[4-(5-Cyano-2-thienyl)phenoxy]tetrahydrofuran-3-yl}propane-2-sulfonamide (PF-04958242)
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-05-14 00:00:00 , DOI: 10.1021/acs.jmedchem.5b00300 Christopher L. Shaffer 1 , Nandini C. Patel 1 , Jacob Schwarz 1 , Renato J. Scialis 1 , Yunjing Wei 1 , Xinjun J. Hou 1 , Longfei Xie 1 , Kapil Karki 1 , Dianne K. Bryce 1 , Sarah M. Osgood 1 , William E. Hoffmann 1 , John T. Lazzaro 1 , Cheng Chang 1 , Dina F. McGinnis 1 , Susan M. Lotarski 1 , JianHua Liu 1 , R. Scott Obach 1 , Mark L. Weber 1 , Laigao Chen 1 , Kenneth R. Zasadny 1 , Patricia A. Seymour 1 , Christopher J. Schmidt 1 , Mihály Hajós 1 , Raymond S. Hurst 1 , Jayvardhan Pandit 1 , Christopher J. O’Donnell 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-05-14 00:00:00 , DOI: 10.1021/acs.jmedchem.5b00300 Christopher L. Shaffer 1 , Nandini C. Patel 1 , Jacob Schwarz 1 , Renato J. Scialis 1 , Yunjing Wei 1 , Xinjun J. Hou 1 , Longfei Xie 1 , Kapil Karki 1 , Dianne K. Bryce 1 , Sarah M. Osgood 1 , William E. Hoffmann 1 , John T. Lazzaro 1 , Cheng Chang 1 , Dina F. McGinnis 1 , Susan M. Lotarski 1 , JianHua Liu 1 , R. Scott Obach 1 , Mark L. Weber 1 , Laigao Chen 1 , Kenneth R. Zasadny 1 , Patricia A. Seymour 1 , Christopher J. Schmidt 1 , Mihály Hajós 1 , Raymond S. Hurst 1 , Jayvardhan Pandit 1 , Christopher J. O’Donnell 1
Affiliation
|
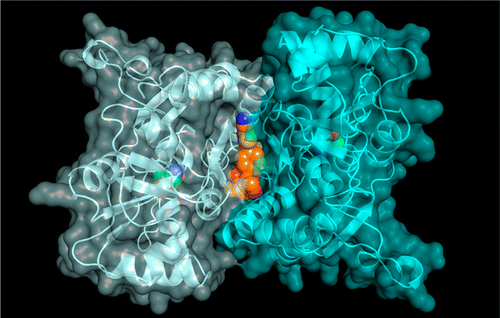
A unique tetrahydrofuran ether class of highly potent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor potentiators has been identified using rational and structure-based drug design. An acyclic lead compound, containing an ether-linked isopropylsulfonamide and biphenyl group, was pharmacologically augmented by converting it to a conformationally constrained tetrahydrofuran to improve key interactions with the human GluA2 ligand-binding domain. Subsequent replacement of the distal phenyl motif with 2-cyanothiophene to enhance its potency, selectivity, and metabolic stability afforded N-{(3S,4S)-4-[4-(5-cyano-2-thienyl)phenoxy]tetrahydrofuran-3-yl}propane-2-sulfonamide (PF-04958242, 3), whose preclinical characterization suggests an adequate therapeutic index, aided by low projected human oral pharmacokinetic variability, for clinical studies exploring its ability to attenuate cognitive deficits in patients with schizophrenia.
中文翻译:

α-氨基-3-羟基-5-羟基-5-甲基-4-异恶唑丙酸(AMPA)受体增强剂N -{(3 S,4 S)-4- [4-(5-Cyano-2-)噻吩基)苯氧基]四氢呋喃-3-基}丙烷-2-磺酰胺(PF-04958242)
使用合理的和基于结构的药物设计,已经确定了一种独特的四氢呋喃醚类高效能α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体增强剂。通过将无环先导化合物转化为构象受限的四氢呋喃,以改善与人GluA2配体结合结构域的关键相互作用,可在药理上增强包含醚连接的异丙基磺酰胺和联苯基的无环先导化合物。随后用2-氰基噻吩代替远端苯基基序以增强其效力,选择性和代谢稳定性,得到N -{(3 S,4 S)-4- [4-(5-氰基-2-噻吩基)苯氧基]四氢呋喃-3-基}丙-2-磺酰胺(PF-04958242,3),其临床前特征表明在预期的低水平人体口服药代动力学变异性的辅助下具有足够的治疗指数,用于临床研究,以探索其减轻精神分裂症患者认知功能障碍的能力。
更新日期:2015-05-14
中文翻译:

α-氨基-3-羟基-5-羟基-5-甲基-4-异恶唑丙酸(AMPA)受体增强剂N -{(3 S,4 S)-4- [4-(5-Cyano-2-)噻吩基)苯氧基]四氢呋喃-3-基}丙烷-2-磺酰胺(PF-04958242)
使用合理的和基于结构的药物设计,已经确定了一种独特的四氢呋喃醚类高效能α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体增强剂。通过将无环先导化合物转化为构象受限的四氢呋喃,以改善与人GluA2配体结合结构域的关键相互作用,可在药理上增强包含醚连接的异丙基磺酰胺和联苯基的无环先导化合物。随后用2-氰基噻吩代替远端苯基基序以增强其效力,选择性和代谢稳定性,得到N -{(3 S,4 S)-4- [4-(5-氰基-2-噻吩基)苯氧基]四氢呋喃-3-基}丙-2-磺酰胺(PF-04958242,3),其临床前特征表明在预期的低水平人体口服药代动力学变异性的辅助下具有足够的治疗指数,用于临床研究,以探索其减轻精神分裂症患者认知功能障碍的能力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号