当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Close-up of the Effect of Iron Oxide Type on the Interfacial Interaction between Epoxy and Carbon Steel: Combined Molecular Dynamics Simulations and Quantum Mechanics
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-05-17 00:00:00 , DOI: 10.1021/acs.jpcc.6b03133 Ghasem Bahlakeh 1 , Mehdi Ghaffari 2 , Mohammad Reza Saeb 3 , Bahram Ramezanzadeh 4 , Frank De Proft 5 , Herman Terryn 6
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-05-17 00:00:00 , DOI: 10.1021/acs.jpcc.6b03133 Ghasem Bahlakeh 1 , Mehdi Ghaffari 2 , Mohammad Reza Saeb 3 , Bahram Ramezanzadeh 4 , Frank De Proft 5 , Herman Terryn 6
Affiliation
|
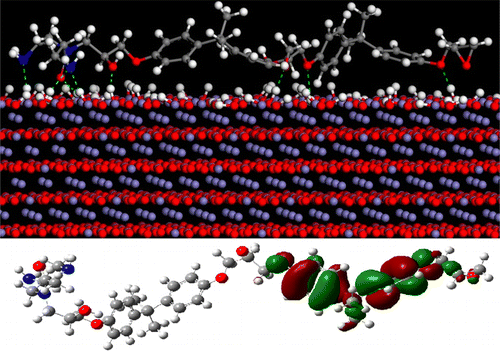
In the present study, the effect of different types of iron oxides, which naturally exist on steel substrate, on the interfacial interaction between an epoxy coating and a carbon steel substrate was studied at the molecular/atomic level by employing molecular dynamics (MD) simulations and quantum mechanics (QM) calculations. Three types of iron oxide, that is, ferrous oxide (FeO), ferric oxide (Fe2O3, hematite), and ferrous ferric oxide (Fe3O4, magnetite), were considered for modeling, and their binding energies were calculated and compared by altering the concentration of hydroxide groups on the surface. To probe the effect of curing agent on interfacial interactions, computations were performed for either uncured or aminoamide-cured epoxy resins. The effect of the acid–base properties of the iron oxide on the molecular bonding was theoretically investigated by imposing diverse iron hydroxide/oxide termination groups. Noticeably, MD and QM calculations confirmed rather well earlier experimental evaluations on iron oxide acid–base properties obtained through X-ray photoelectron spectroscopy measurements in the view of chemisorption of different epoxy compounds. However, the interaction behavior of cured epoxy with hydroxylated iron oxide surfaces was quantified mechanistically in the current work from a closer view. For instance, it was found that aminoamide-cured epoxy was adsorbed on all oxide substrates through a mechanism of electrostatic and donor–acceptor interactions, with binding energies of −113.6, −1035.9, and −304.4 kcal/mol respectively assigned to FeO, Fe2O3, and Fe3O4 iron oxides. In the case of a hydroxylated surface, aminoamide-conjugated epoxy adhesive was found in the hydrogen bond interface as well, which was evidence of strengthened binding at a surface populated by hydroxide groups. Moreover, theoretical explorations showed that the type of covalent linkage between the curing molecule and epoxy resin governs the extent of cured epoxy adhesion to the surface of iron oxide.
中文翻译:

氧化铁类型对环氧树脂和碳钢之间界面相互作用的影响的特写:分子动力学模拟和量子力学的组合
在本研究中,通过使用分子动力学(MD)模拟,在分子/原子水平上研究了钢基底上自然存在的不同类型的氧化铁对环氧涂层与碳钢基底之间的界面相互作用的影响。和量子力学(QM)计算。三种类型的氧化铁,即氧化亚铁(FeO),三氧化二铁(Fe 2 O 3,赤铁矿)和三氧化二铁(Fe 3 O 4(磁铁矿)进行建模,并通过改变表面上的氢氧根基团的浓度来计算和比较它们的结合能。为了探究固化剂对界面相互作用的影响,对未固化或氨基酰胺固化的环氧树脂进行了计算。理论上通过施加各种氢氧化铁/氧化物终止基团,研究了氧化铁的酸碱性质对分子键合的影响。值得注意的是,考虑到不同环氧化合物的化学吸附作用,MD和QM的计算证实了较早的实验评估,该评估是通过X射线光电子能谱测量获得的氧化铁酸碱性质。然而,在近距离的工作中,通过机械方法定量地定量了固化的环氧树脂与羟基化的氧化铁表面的相互作用。例如,发现氨基酰胺固化的环氧树脂通过静电和施主-受主相互作用的机制吸附在所有氧化物基质上,结合能分别为-113.6,-1035.9和-304.4 kcal / mol分配给FeO,Fe2 O 3和Fe 3 O 4的氧化铁。在羟基化表面的情况下,在氢键界面上也发现了氨基酰胺共轭环氧胶粘剂,这证明在由氢氧根组成的表面上结合力增强。此外,理论探索表明,固化分子与环氧树脂之间的共价键的类型决定了固化后的环氧树脂与氧化铁表面的粘附程度。
更新日期:2016-05-17
中文翻译:

氧化铁类型对环氧树脂和碳钢之间界面相互作用的影响的特写:分子动力学模拟和量子力学的组合
在本研究中,通过使用分子动力学(MD)模拟,在分子/原子水平上研究了钢基底上自然存在的不同类型的氧化铁对环氧涂层与碳钢基底之间的界面相互作用的影响。和量子力学(QM)计算。三种类型的氧化铁,即氧化亚铁(FeO),三氧化二铁(Fe 2 O 3,赤铁矿)和三氧化二铁(Fe 3 O 4(磁铁矿)进行建模,并通过改变表面上的氢氧根基团的浓度来计算和比较它们的结合能。为了探究固化剂对界面相互作用的影响,对未固化或氨基酰胺固化的环氧树脂进行了计算。理论上通过施加各种氢氧化铁/氧化物终止基团,研究了氧化铁的酸碱性质对分子键合的影响。值得注意的是,考虑到不同环氧化合物的化学吸附作用,MD和QM的计算证实了较早的实验评估,该评估是通过X射线光电子能谱测量获得的氧化铁酸碱性质。然而,在近距离的工作中,通过机械方法定量地定量了固化的环氧树脂与羟基化的氧化铁表面的相互作用。例如,发现氨基酰胺固化的环氧树脂通过静电和施主-受主相互作用的机制吸附在所有氧化物基质上,结合能分别为-113.6,-1035.9和-304.4 kcal / mol分配给FeO,Fe2 O 3和Fe 3 O 4的氧化铁。在羟基化表面的情况下,在氢键界面上也发现了氨基酰胺共轭环氧胶粘剂,这证明在由氢氧根组成的表面上结合力增强。此外,理论探索表明,固化分子与环氧树脂之间的共价键的类型决定了固化后的环氧树脂与氧化铁表面的粘附程度。































 京公网安备 11010802027423号
京公网安备 11010802027423号