当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of the Privileged 8-Arylmenthol Class by Radical Arylation of Isopulegol
Organic Letters ( IF 4.9 ) Pub Date : 2016-05-13 00:00:00 , DOI: 10.1021/acs.orglett.6b01047 Steven W. M. Crossley 1 , Ruben M. Martinez 1 , Sebastián Guevara-Zuluaga 1 , Ryan A. Shenvi 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-05-13 00:00:00 , DOI: 10.1021/acs.orglett.6b01047 Steven W. M. Crossley 1 , Ruben M. Martinez 1 , Sebastián Guevara-Zuluaga 1 , Ryan A. Shenvi 1
Affiliation
|
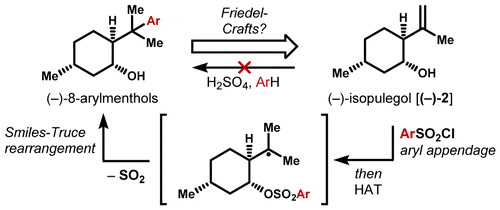
Hydrogen atom transfer (HAT) circumvents a disfavored Friedel–Crafts reaction in the derivatization of the inexpensive monoterpene isopulegol. A variety of readily prepared aryl and heteroaryl sulfonates undergo a formal hydroarylation to form 8-arylmenthols, privileged scaffolds for asymmetric synthesis, as typified by 8-phenylmenthol. High stereoselectivity is observed in related systems. This use of HAT significantly extends the chiral pool from the inexpensive monoterpene isopulegol.
中文翻译:

异戊二醇的自由基芳基化反应合成8-芳基醇类特权化合物
氢原子转移(HAT)避免了廉价的单萜异胡薄荷醇的衍生化中不利的Friedel-Crafts反应。各种易于制备的芳基和杂芳基磺酸盐经过正式的氢芳基化反应生成8-芳基薄荷醇,这是用于不对称合成的有优势的支架,以8-苯基薄荷醇为代表。在相关系统中观察到高立体选择性。HAT的这种使用极大地扩展了廉价的单萜异胡薄荷醇的手性库。
更新日期:2016-05-13
中文翻译:

异戊二醇的自由基芳基化反应合成8-芳基醇类特权化合物
氢原子转移(HAT)避免了廉价的单萜异胡薄荷醇的衍生化中不利的Friedel-Crafts反应。各种易于制备的芳基和杂芳基磺酸盐经过正式的氢芳基化反应生成8-芳基薄荷醇,这是用于不对称合成的有优势的支架,以8-苯基薄荷醇为代表。在相关系统中观察到高立体选择性。HAT的这种使用极大地扩展了廉价的单萜异胡薄荷醇的手性库。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号