当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Active sites of ligand-protected Au25 nanoparticle catalysts for CO2 electroreduction to CO
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-05-11 13:59:28 , DOI: 10.1063/1.4948792 Dominic R. Alfonso 1 , Douglas Kauffman 1 , Christopher Matranga 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-05-11 13:59:28 , DOI: 10.1063/1.4948792 Dominic R. Alfonso 1 , Douglas Kauffman 1 , Christopher Matranga 1
Affiliation
|
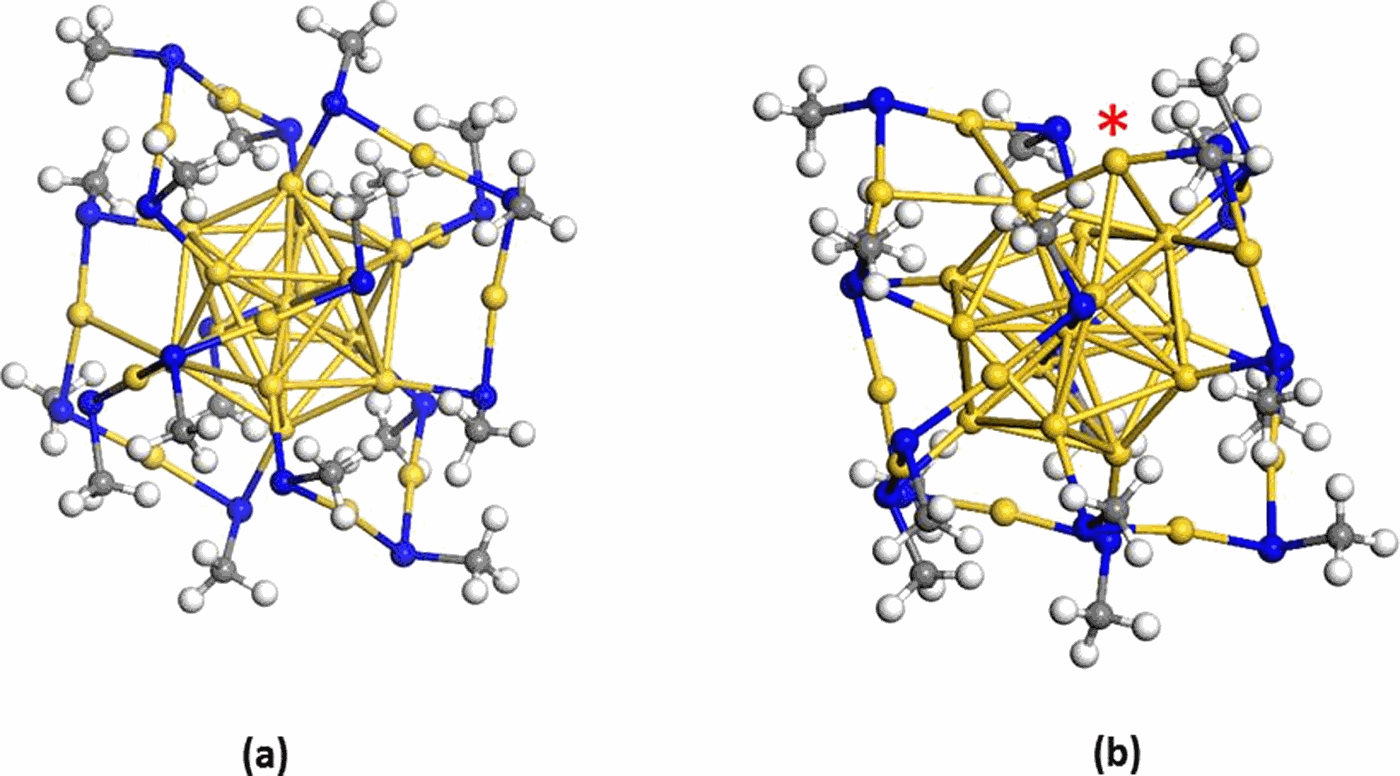
Recent experimental studies have reported the electrochemical reduction of carbon dioxide (CO2) into CO at atomically precise negatively charged Au25−nanoclusters. The studies showed CO2 conversion at remarkably low overpotentials, but the exact mechanisms and nature of the active sites remain unclear. We used first-principles density functional theory and continuum solvation models to examine the role of the cluster during electrochemical CO2 reduction and analyze the free energies of proposed intermediate species. Contrary to previous assumptions, our results show that the fully ligand protected cluster is not an active CO2 reduction catalyst because formation of the crucial carboxyl intermediate required very high electrochemical potentials. Instead, our calculations suggest that the reduction process likely occurs on a dethiolated gold site, and adsorbed carboxyl intermediate formation was significantly stabilized at dethiolated gold sites. These findings point to the crucial role of exposed metal sites during electrochemical CO2 reduction at goldnanocluster catalysts.
中文翻译:

配体保护的Au25纳米颗粒催化剂的活性位点,用于将CO2电还原为CO
最近的实验研究中报告的电化学还原二氧化碳(CO 2)转换成CO在原子级精确带负电荷的Au 25 -纳米簇。研究表明,CO 2转化的过电位极低,但活性位点的确切机理和性质仍不清楚。我们使用第一原理密度泛函理论和连续溶剂化模型检查了簇在电化学CO 2还原过程中的作用,并分析了所提出的中间物种的自由能。与以前的假设相反,我们的结果表明,完全被配体保护的簇不是活性CO 2还原催化剂,因为关键的羧基中间体的形成需要很高的电化学势。相反,我们的计算表明还原过程可能发生在脱硫金位置,并且吸附的羧基中间体的形成在脱硫金位置显着稳定。这些发现指出了在金纳米簇催化剂上电化学CO 2还原期间暴露的金属位点的关键作用。
更新日期:2016-05-12
中文翻译:

配体保护的Au25纳米颗粒催化剂的活性位点,用于将CO2电还原为CO
最近的实验研究中报告的电化学还原二氧化碳(CO 2)转换成CO在原子级精确带负电荷的Au 25 -纳米簇。研究表明,CO 2转化的过电位极低,但活性位点的确切机理和性质仍不清楚。我们使用第一原理密度泛函理论和连续溶剂化模型检查了簇在电化学CO 2还原过程中的作用,并分析了所提出的中间物种的自由能。与以前的假设相反,我们的结果表明,完全被配体保护的簇不是活性CO 2还原催化剂,因为关键的羧基中间体的形成需要很高的电化学势。相反,我们的计算表明还原过程可能发生在脱硫金位置,并且吸附的羧基中间体的形成在脱硫金位置显着稳定。这些发现指出了在金纳米簇催化剂上电化学CO 2还原期间暴露的金属位点的关键作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号