当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed carbonylative (3+1+2) cycloadditions of amino-substituted cyclopropanes and alkynes: reaction development and increased efficiencies using a cationic rhodium system
Tetrahedron ( IF 2.1 ) Pub Date : 2016-05-07 11:16:11 Megan H. Shaw, William G. Whittingham, John F. Bower
Tetrahedron ( IF 2.1 ) Pub Date : 2016-05-07 11:16:11 Megan H. Shaw, William G. Whittingham, John F. Bower
|
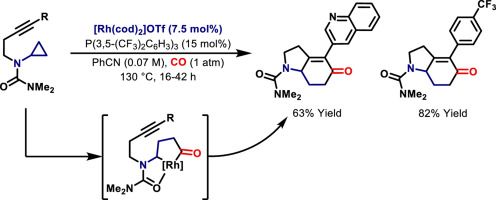
Urea-directed carbonylative insertion of Rh(I)-catalysts into one of the two proximal C–C bonds of aminocyclopropanes generates rhodacyclopentanone intermediates. These are trapped by N-tethered alkynes to provide a (3+1+2) cycloaddition protocol that accesses N-heterobicyclic enones. Stoichiometric studies on a series of model rhodacyclopentanone complexes outline key structural features and provide a rationale for the efficacy of urea directing groups. A comprehensive evaluation of cycloaddition scope and a ‘second generation’ cationic Rh(I)-system, which provides enhanced yields and reaction rates for challenging substrates, are presented.
中文翻译:

氨基取代的环丙烷和炔烃的直接羰基化(3 + 1 + 2)环加成反应:使用阳离子铑体系进行反应开发并提高效率
尿素定向的Rh(I)催化剂羰基插入氨基环丙烷的两个近端C–C键之一会生成Rhodacyclopentanone中间体。这些被N-拴链的炔烃捕获以提供(3 + 1 + 2)环加成协议,该协议可访问N-杂环双环烯酮。对一系列模型若丹环戊酮配合物的化学计量研究概述了关键的结构特征,并为尿素指导基团的功效提供了理论依据。介绍了对环加成范围和“第二代”阳离子Rh(I)系统的综合评估,该系统可提高具有挑战性的底物的产率和反应速率。
更新日期:2016-05-08
中文翻译:

氨基取代的环丙烷和炔烃的直接羰基化(3 + 1 + 2)环加成反应:使用阳离子铑体系进行反应开发并提高效率
尿素定向的Rh(I)催化剂羰基插入氨基环丙烷的两个近端C–C键之一会生成Rhodacyclopentanone中间体。这些被N-拴链的炔烃捕获以提供(3 + 1 + 2)环加成协议,该协议可访问N-杂环双环烯酮。对一系列模型若丹环戊酮配合物的化学计量研究概述了关键的结构特征,并为尿素指导基团的功效提供了理论依据。介绍了对环加成范围和“第二代”阳离子Rh(I)系统的综合评估,该系统可提高具有挑战性的底物的产率和反应速率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号