当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-03-18 00:00:00 , DOI: 10.1021/acs.chemmater.5b00339
Chao Xu 1 , Fredrik Lindgren 1 , Bertrand Philippe 2 , Mihaela Gorgoi 3 , Fredrik Björefors 1 , Kristina Edström 1 , Torbjörn Gustafsson 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-03-18 00:00:00 , DOI: 10.1021/acs.chemmater.5b00339
Chao Xu 1 , Fredrik Lindgren 1 , Bertrand Philippe 2 , Mihaela Gorgoi 3 , Fredrik Björefors 1 , Kristina Edström 1 , Torbjörn Gustafsson 1
Affiliation
|
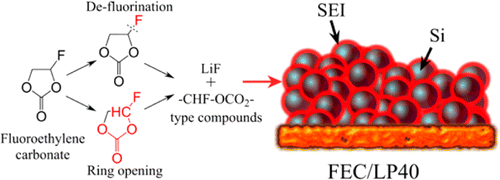
Silicon as a negative electrode material for lithium-ion batteries has attracted tremendous attention due to its high theoretical capacity, and fluoroethylene carbonate (FEC) was used as an electrolyte additive, which significantly improved the cyclability of silicon-based electrodes in this study. The decomposition of the FEC additive was investigated by synchrotron-based X-ray photoelectron spectroscopy (PES) giving a chemical composition depth-profile. The reduction products of FEC were found to mainly consist of LiF and −CHF–OCO2-type compounds. Moreover, FEC influenced the lithium hexafluorophosphate (LiPF6) decomposition reaction and may have suppressed further salt degradation. The solid electrolyte interphase (SEI) formed from the decomposition of ethylene carbonate (EC) and diethyl carbonate (DEC), without the FEC additive present, covered surface voids and lead to an increase in polarization. However, in the presence of FEC, which degrades at a higher reduction potential than EC and DEC, instantaneously a conformal SEI was formed on the silicon electrode. This stable SEI layer sufficiently limited the emergence of large cracks and preserved the original surface morphology as well as suppressed the additional SEI formation from the other solvent. This study highlights the vital importance of how the chemical composition and morphology of the SEI influence battery performance.
中文翻译:

锂离子电池用硅阳极的改进性能:了解碳酸氟乙烯酯作为一种有效的电解质添加剂的表面改性机理
硅作为锂离子电池的负极材料,由于其理论容量高而备受关注,而碳酸氟代亚乙酯(FEC)被用作电解质添加剂,在本研究中显着提高了硅基电极的循环性。通过基于同步加速器的X射线光电子能谱(PES)研究了FEC添加剂的分解,从而给出了化学成分的深度分布。发现FEC的还原产物主要由LiF和-CHF-OCO 2型化合物组成。此外,FEC影响了六氟磷酸锂(LiPF 6)分解反应,并可能抑制盐进一步降解。在不存在FEC添加剂的情况下,由碳酸亚乙酯(EC)和碳酸二乙酯(DEC)分解形成的固体电解质中间相(SEI)覆盖了表面空隙并导致极化增加。然而,在FEC的存在下,其以比EC和DEC更高的还原电位降解,在硅电极上瞬间形成保形的SEI。这种稳定的SEI层足以限制大裂纹的出现,并保留了原始的表面形态,并抑制了其他溶剂形成的SEI。这项研究强调了SEI的化学成分和形态如何影响电池性能的至关重要。
更新日期:2015-03-18
中文翻译:

锂离子电池用硅阳极的改进性能:了解碳酸氟乙烯酯作为一种有效的电解质添加剂的表面改性机理
硅作为锂离子电池的负极材料,由于其理论容量高而备受关注,而碳酸氟代亚乙酯(FEC)被用作电解质添加剂,在本研究中显着提高了硅基电极的循环性。通过基于同步加速器的X射线光电子能谱(PES)研究了FEC添加剂的分解,从而给出了化学成分的深度分布。发现FEC的还原产物主要由LiF和-CHF-OCO 2型化合物组成。此外,FEC影响了六氟磷酸锂(LiPF 6)分解反应,并可能抑制盐进一步降解。在不存在FEC添加剂的情况下,由碳酸亚乙酯(EC)和碳酸二乙酯(DEC)分解形成的固体电解质中间相(SEI)覆盖了表面空隙并导致极化增加。然而,在FEC的存在下,其以比EC和DEC更高的还原电位降解,在硅电极上瞬间形成保形的SEI。这种稳定的SEI层足以限制大裂纹的出现,并保留了原始的表面形态,并抑制了其他溶剂形成的SEI。这项研究强调了SEI的化学成分和形态如何影响电池性能的至关重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号